A Mini Review of Catalytic Reducing Nitrogen to Ammonia under Ambient Conditions
Jianjun Yang1,2*
1Department of Chemical Engineering, School of Environmental Science and Engineering, China
2Key Laboratory of Subsurface Hydrology and Ecological Effects in Arid Region Chang’an University, China
Submission: January 28, 2019Published: February 26, 2019
*Corresponding author: Jianjun Yang, Department of Chemical Engineering, School of Environmental Science and Engineering, China
How to cite this article: Jianjun Yang. A Mini Review of Catalytic Reducing Nitrogen to Ammonia under Ambient Conditions. Organic & Medicinal Chem IJ. 2019; 8(2): 555732 DOI: 10.19080/OMCIJ.2019.08.555732
>Abstract
Ammonia(NH3) has played an essential role in meeting the increasing demand for food and the worldwide need of nitrogenous fertilizer since 1913. Unfortunately, the traditional Haber-Bosch process for producing NH3 from nitrogen(N2) is a high energy-consumption process. Under ambient conditions catalytic reducing N2 to NH3 is an attractive and promising alternative approach which would emerge huge opportunity to directly provide nitrogenous fertilizers in agricultural fields as need in a distributed manner. In this review, some research findings showed alternative, available, sustainable NH3 production processes from N2 in the presence of electro-catalysts and photo-catalysts under ambient conditions
Keywords: Catalyst Nitrogen Ammonia Ambient condition
Introduction
As one of the most important chemicals to our planet, NH3 has played an essential role in meeting the growing demand for food and the worldwide need of nitrogenous fertilizer since 1913 [1]. The total worldwide NH3 production exceeded 140 million tons, and demand for NH3 continues to grow in 2014 [2], and about 80% of the NH3 product usually is used as nitrogenous fertilizer. Fritz Haber discovered that NH3 could be directly synthesized by reacting atmospheric N2 with hydrogen in the temperature range of 400-500℃ and at pressures of 130-170bar [3], and Carl Bosch subsequently developed it on an industrial scale [4]. As the most important invention of the 20th century, the Haber-Bosch process, a thermo-chemical catalytic conversion technology, is a primary choice of industrial production of NH3 for human beings until now. According to the Haber-Bosch process, NH3 is produced from the reaction:
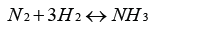
The Haber-Bosch process reacts the pure feed gases at high temperatures and pressures, requiring energy input of around 485 kJ mol-1of N2 and almost 2% of global energy consumption [5]. The high dissociation energy of triply bonded N2 molecule (911 kJ mol-1) presents a significant activation energy barrier, however, the negative entropy (ΔH300= -46.35 kJ mol-1) of the reaction dictates that N2 could be converted to NH3 at lower temperatures [6]. There are several advantages for NH3 synthesis at low temperatures [7,8]. Firstly, the reaction of producing NH3 from N2 with dihydrogen is spontaneous. Secondly, the proton conductivity of low-temperature electrolytes has more excellent behaves than the other temperature ones. Thirdly, the reaction kinetics of NH3 production processes is extremely slow when the operating temperatures are below 100℃. It is Kordali et. al. [9] who firstly reported that NH3 was produced from N2 and water using a Nafion electrolyte at low temperature in 2000. In additional, it would be a key joint to build foundational principles of designing new efficient catalysts for sustainable NH3 synthesis production process. The new methods of catalyst design need us to understand the catalytic mechanisms by integrating theory and experiment of discovering the active, scalable, selective, long-lived efficient catalysts for sustainable NH3 synthesis.
Therefore, how to activate the N≡N bond to produce NH3 with less fossil energy consumption is great opportunity and challenges for chemists. Although researchers tried to develop the artificial catalysts to facilitate the reaction at more ambient conditions and there are many new approaches, the nowadays used industrial catalysts are extremely similar to the original one discovered by Mittasch [10] in 1910s. In this review, some research findings showed alternative, available, sustainable NH3 production processes from N2 in the presence of electro-catalysts, photo-catalysts and analogous catalysts of nitrogenases metalloclusters under ambient conditions.
Discussion
Recently, electro-catalysis depending on renewable electricity produced from renewable energy (such as wind or solar) has played an increasingly role in the NH3 synthesis at ambient temperature(T<100℃) [2,7,8,11]. Some novel functional materials as catalysts used in typical N2 reductions were explored by researchers [12].
Electro-Driven Catalytic Reducing N2 to NH3
Under ambient conditions, Liu et al. [13,14] developed Sm1.5Sr0.5MO4(M=Ni, Co, Fe) and SmFe0.7Cu0.3-xNix- O3(x=0–0.3) as electro-catalysts for NH 3 synthesis with Nafion used as proton solid electrolyte. Such systems, while impressive in current efficiency, require careful control experiments due to low overall yields and observed ambient NH3 absorption by polymer electrolyte membranes. Further, researchers have taken their efforts to develop ideal electro-catalysts for improving N2 reduction rates and the NH3 yield. Impressively, SmFe0. 7Cu0.1Ni0.2O3 showed the record-high NH3 production rate of 1.1×10-8 mol·s-1·cm-2 at 80℃. The NH3 yield was up to 8.7×10–9 mol·s-1·cm-2 in the present of electro-catalyst SmBaCuMO5+i (M=Fe, Co,Ni) at 80℃ and 2.5V [15]. However, there are still big spaces to improve the NH3 yield which currently are not enough to the future industry application.
An alternative strategy would like to consider using metal nitrides as electro-catalyst cathodes for N2 reduction except pure metals. Respectively at potentials of -0.76V and -0.51V, producing NH3 from N2 took place on the surface of Zr N and VN as electro-catalyst cathodes which were not covered by adsorbed H-atoms [16], the same to Nb N and CrN [17]. The theoretical analysis of Abghoui et al. [18-20] predicted stable operation and Faraday Efficiency(FE) higher than 75% for V, Cr, Nb and Zr mono-nitrides at applied bias between 0.5 and 0.76V. Unfortunately, there would not be such nitride materials which were tested experimentally like above mentioned for NH3 synthesis [21]. Beside mono-nitrides, binary nitrides such as Co3Mo3N are amongst the most active electro-catalysts for NH3 synthesis [22]. A thorough investigation of their electro-catalytic behaves should be performed in real NH3 synthesis situations.
These reports show that there was a universal phenomenon that these electro-catalysts often contain big proportions of oxides on their surfaces, which is harmful to keep their normal conductivity and could significantly reduce their catalytic efficiency. Actually, an ideal electro-catalyst should have the capacities of high catalytic activity and excellent electronic conductivity at ambient conditions and could suppress the hydrogen evolution reaction. The electro-catalytic NH3 synthesis is still under experiment stage, huge challenges are still faced the lack of the solid oxide electrolyte materials [23]. Future research should focus on theory-guided screening and discovery of new NH3 catalysts, as well as developing novel methods for NH3 synthesis at low temperatures and pressures. One important issue on the successful demonstration of NH3 beingfrom reducing N2 and Overcoming N2 and NH3 contamination should be done with 15 N2 dinitrogen, FTIR analysis and choice of system materials.
Photo-Driven Catalytic Reducing N2 to NH3
With the global population increasing, the photon-driven N2 fixation technologies will become very critical to capture solar energy and produce nitrogenous fertilizers [24]. In the late 1970s, Schrauzer and Guth discovered that TiO2 powders doped with Fe2O3 could catalyze the reduction of N2 to NH3 under ambient conditions [25,26]. Since then, TiO2 became the workhorse photo-catalyst for subsequent studies, and CuCl, WO3 and FeOx as possible photo-catalysts for NH3 synthesis have been investigated [27,28]. Maybe, surface oxygen defects of titanium metal caused by high temperatures could play an important role in the photo-driven catalytic reduction of N2 to NH3. Although there is not an insight regarding the atomic scale phenomena on photo-catalytic N2 reduction, the TiO2 crystal polymorph and its iron impurity concentration are identified to be the key factors to underlay photo-catalytic performance.
Co-catalysts, such as Co, Mo, Ni, Ru and Pt dopants, have been added to the titanium-based materials for increasing the NH3 yield [24,29]. Transition metal dopants as electron sinks in titanium photo-catalysts could minimize the probability for carrier recombination to promote greater NH3 yields. Dopants inducing defect states could assist in charge separation of photogenerated electrons and holes but are not a part of the active site for N2 dissociation. Therefore, the conduction band position of photo-catalysts (Figure 1) should be more negative than the reduction potential of the N2 hydrogenation, as well as the valance band should be more positive than the oxygen evolution potential [30].
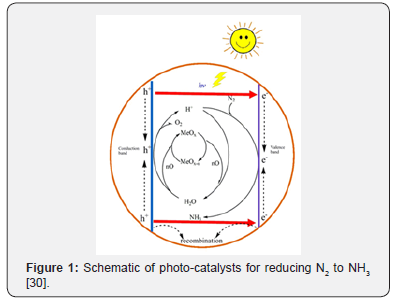
The partial current densities for photo-catalytic producing NH3 are lower 3 to 5 orders of magnitude than the other electrodriven NH3 production processes. To solve the problem, maybe the efficient photo-catalysts attached to electrodes would show us an amazing result of the higher NH3 yields. In additional, likeelectrochemical NH3 synthesis, photo-catalytic approaches must employ proper methods and controls.
Conclusion
Under ambient conditions, some seminal literatures have been reviewed on describing the electro-catalytic NH3 synthesis from commonly available feedstock such as purified H2 and N2. The electro-catalytic NH3 synthesis is still under experiment stage due to the lack of the suitable electrolyte materials as electro-catalysts with high catalytic activity, excellent electronic conductivity, and hydrogen evolution reaction. Transition metals which can achieve selective N2 reduction maybe are good choice for further researches. Additionally, it is with the high-energy UV light that photo-catalytic NH3 synthesis from N2 could overcome the N≡N band energy barrier under ambient conditions. Currently, titanium oxide with transition metal dopants is a not bad choice in practice. Future studies should encourage investigating on how N2 reduction happen on the photo-catalysts with the application of modern computational and experimental techniques from molecular level viewpoint
Acknowledgment
This work was supported by the China Scholarship Council (No. 201706565033).
Conflict of Interest
The author has declared that any economic interest or any conflict of interest exists.
References
- T Kandemir, Schuster ME, Senyshyn A, Behrens M, Schlögl R (2013) The Haber-Bosch Process Revisited: On the Real Structure and Stability of “Ammonia Iron” under Working Conditions. Angew Chem Int Ed 52(48): 12723-12726.
- Shipman MA, Symes MD (2017) Recent progress towards the electro-synthesis of ammonia from sustainable resources. Catalysis Today 286: 57-68.
- Haber F, Leiser R (1918) Method and apparatus for testing gases, US1269599A.
- Carl Bosch (1932) The development of the chemical high-pressure method during the establishment of the new ammonia industry, Nobel Lectures:197-235.
- Erisman JW, Bleeker A, Galloway J, Sutton M S(2007) Reduced nitrogen in ecology and the environment, Environmental Pollution 150(1): 140-149.
- Giddey S, Badwal SPS, Kulkarni A (2013) Review of electrochemical ammonia production technologies and materials. International journal of hydrogen energy 38: 14576-14594.
- Garagounis I, Kyriakou V, Skodra A, Vasileiou E, Stoukides M (2014) Electrochemical synthesis of ammonia in solid electrolyte cells. Front Energy Res 2: 1-10.
- IA Amar, R Lan, CTG Petit SW Tao (2011) Solid-state electrochemical synthesis of ammonia: a review J Solid State Electro chem 15: 1845-1860.
- Kordali V, Kyriakou G, Lambrou C (2000) Electrochemical synthesis of ammonia at atmospheric pressure and low temperature in a solid polymer electrolyte cell. Chem Commum 17(2000): 1673-1674.
- Schlögl R (2003) Catalytic Synthesis of Ammonia-A “Never‐Ending Story”? Angew Chem Int Ed Engl 42(18): 2004-2008.
- Giddey S, Badwal SPS, Kulkarni A (2013) Review of electrochemical ammonia production technologies and materials, International J. Hydrogen Energy 38: 14576-14594.
- Kyriakou V, Garagounis I, Vasileiou E, Vourros A, Stoukides M (2017) Progress in the Electrochemical Synthesis of Ammonia, Catalysis Today 286: 2-13.
- Xu GC, Liu RQ, Wang J (2009) Electrochemical synthesis of ammonia using a cell with a Nafion membrane and SmFe0.7Cu0.3-xNi xO3 (x=0−0.3) cathode at atmospheric pressure and lower temperature. Sci China Ser B: Chem 52(8): 1171-1175.
- Liu RQ , Xu G (2010) Comparison of Electrochemical Synthesis of Ammonia by Using Sulfonated Polysulfone and Nafion Membrane with Sm1.5Sr0.5NiO4. Chin J Chem 28(2): 139-142.
- Hang ZF, Zhong ZP, Liu RQ (2010) Cathode catalysis performance of SmBaCuMO5 (M=Fe, Co, Ni) in ammonia synthesis, Journal of Rare Earths 28(4): 556-559.
- Abghoui Y, Garden AL, Hlynsson VF, Björgvinsdóttir S, Ólafsdóttir H, Skúlason E (2015) Enabling electrochemical reduction of nitrogen to ammonia at ambient conditions through rational catalyst design, Phys Chem Chem Phys 17: 4909-4918.
- Abghoui Y, Garden AL, Howalt JG, Vegge T, Skúlason E (2016) Electroreduction of Nitrogen to Ammonia at Ambient Conditions on Mononitrides of Zr, Nb, Cr, and V: A DFT Guide for Experiments, ACS Catal 6(2): 635-646.
- Ivana M, Fernando HG, Neil JH (2014) Electro-reduction of nitrogen on molybdenum nitride: structure, energetics, and vibrational spectra from DFT, Phys Chem Chem Phys 16: 3014-3026.
- Hlynsson VF, Skúlason E, Garden AL (2014) A systematic, first-principles study of the structural preference and magnetic properties of mononitrides of the d-block metals. J Alloys Compd 603(2014): 172-179.
- Michalsky R, Avram AM, Peterson BA, Pfromm PH, Peterson AA (2015) Chemical looping of metal nitride catalysts: low-pressure ammonia synthesis for energy storage, Chem Sci 6(7): 3965-3974.
- Michael A, Shipman, Mark D, Symes (2017) Recent progress towards the electrosynthesis of ammonia from sustainable resources, Catalysis Today 286: 57-68.
- Jacobsen CJH (2000) Novel class of ammonia synthesis catalysts Chem Commun 1057-1058.
- Xinghua Guo, Yunpei Zhu, Tianyi Ma (2017) Lowering reaction temperature: Electrochemical ammonia synthesis by coupling various electrolytes and catalysts, Journal of Energy Chemistry 26:1107-1116.
- Andrew J, Medford, Marta C, Hatzell (2017) Photon-Driven Nitrogen Fixation: Current Progress, Thermodynamic Considerations, and Future Outlook, ACS Catal 7: 2624-2643.
- Schrauzer GN, Guth TD (1977) Photolysis of Water and Photoreduction of Nitrogen on Titanium Dioxide. J Am Chem Soc 99(22): 7189-7193.
- Schrauzer G, Guth T, Palmer M (1979) In Solar Energy: Chemical Conversion and Storage. J Nitrogen reducing solar cells, The Humana Press: Clifton NJ: 261-269.
- Yamazoe S, Yasuyuki M, Kentaro T, Yutaka H, Tetsuya S, et al. (2008) Promotion effect of tungsten oxide on photo-assisted selective catalytic reduction of NO with NH3 over TiO2, Applied Catalysis B: Environmental 83(1-2): 123-130.
- Tennakone K, Punchihewa S, Tantrigoda R (1989) Nitrogen photoreduction with cuprous chloride coated hydrous cuprous oxide, Solar Energy Materials 18(3-4): 217-221.
- Ranjit K, Varadarajan T, Viswanathan B (1996) Photocatalytic reduction of dinitrogen to ammonia over noble-metal-loaded TiO2, Journal of Photochemistry and Photobiology A: Chemistry 96(1-3): 181-185.
- Jianjun Yang (2018) Progress of metal oxide(sulfide) based photo-catalytic materials for reducing nitrogen to ammonia. J Chem 2018: 1-8.






























