Treatment of Refinery and Petrochemical Wastewater Using Banana Peel as A Natural Coagulant
Nwanisobi Gloria* and lfeoma Obiora- okafo
Department of Chemical Engineering, Madonna University, Nigeria
Submission: August 15, 2018; Published: September 10, 2018
*Corresponding author: Nwanisobi Gloria, Department of Chemical Engineering, Faculty of Engineering and Technology, Madonna University (Akpugo Campus) Enugu State, Nigeria, Email: glochinwa4real@yahoo.com
How to cite this article: Nwanisobi G. Treatment of Refinery and Petrochemical Wastewater Using Banana Peel as A Natural Coagulant. Organic & Medicinal Chem IJ. 2018; 7(5): 555725. DOI: 10.19080/OMCIJ.2018.07.555725
Abstract
The investigation into the ability of a natural coagulant banana peel to treat refinery and petrochemical waste water has been studied. The waste generated from various activities in these industries is usually disposed and promotes environmental degradation. Coagulation method was used in the treatment of the wastewater. Results reveal that turbidity of the waste water was 39.3%. The banana peel had a turbidity removal efficiency of 71.9% at pH of 8.0.
Keywords:Natural Coagulant; Turbidity Removal Efficiency; Banana peels
Introduction
Coagulation and flocculation processes are physical-chemical methods that are widely used in the treatment of wastewater [1]. In the treatment of wastewater, coagulation has been used in the past with the aim of removing colloidal impurities. It has been noted that in the developing countries more than 1.6 million people are using the unhygienic water & among them most of the people suffers from diarrhea and other water related diseases [2]. Wastewater treatment techniques that are widely used are chemical precipitation, lime coagulation, ion exchange, reverse osmosis and solvent extraction [3]. Other bio adsorbent prepared from banana peels has been reported for the removal of chromium, cadmium and copper ions from aqueous solution [4]. Today, the prime concern of the environmental engineers is how to lower the coagulants and flocculants cost and to improve the characteristics of the produced sludge for safe utilization [5]. Coagulants play a major role in the treatment of water, wastewater and in the treatment and disposal of sludge [6]. Although many water treatment methods have been utilized, most of them are expensive [7]. The aim of this research is to investigate the ability of low cost banana peel to extract contaminants and heavy metals off refinery and petrochemical waste water.
Materials and Methods
The waste water was collected from Warri refining and Nigeria National Petroleum Co-operation (NNPC) petrochemical company in Warri, Delta State of Nigeria and were characterized at thermocouple temperature. Matured banana bunch containing 63 seeds were purchased from Agbani market, Enugu State, Nigeria. The peeled banana was cut into tiny bits and then washed with distilled water. This was sundried for 20days. The dried peels were pulverized and sieved with a mesh size of 0.85mm to obtain a uniform particle size.
Extraction of Active Components in the Coagulant
The peels were grounded and sieved with a 0.85mm mesh size to make the particles finer for better extraction purposes. 2g of the coagulant precursor was weighed and added to 100ml of distilled water to make 2% suspension. The suspension was stirred using a magnetic stirrer for 20minutes at room temperature (29oC) before filtration. This experiment was repeated three times.
Coagulation Experiment
Optimum pH: Place 10ml of the active component, 10ml of buffer 2 and 20ml of wastewater into a beaker. Using a turbidity meter, record your initial value. Stir rapidly at 150rpm for 4minutes and slowly stir at 50 rpm for 20minutes at room temperature (29oC). Pour the mixture into a sample bottle and allow settling for 30minutes. Place the supernatant portion into a turbid meter and record your final value. Calculate the turbidity efficiency using equation 1 [8]. Repeat the following procedure using buffers 4, 6, 8, 10 keeping the other parameters constant.
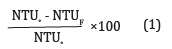
Suitable Coagulant Dosage: Place 5ml of the active component, 10ml of buffer 8 and 20ml of waste water in a beaker, using a turbid meter, record your initial value. Stir rapidly for 4minutes, slowly stir for 20minutes at room temperature (29oC). Pour the mixture into a sample bottle and allow settling for 30minutes. Place the supernatant portion into a turbid meter and record your final value. Calculate the turbidity efficiency using equation 1. Repeat the following procedure using 10ml- 25ml in 5.0 steps of the coagulant dosage respectively while keeping other parameters constant.
Best Settling Time: Place 20ml of the coagulant dosage, 10ml of buffer 8 and 20ml of waste water into a beaker, using a turbid meter record your initial value. Stir rapidly for 4minutes, slowly stir for 20minutes at room temperature (29oC). Pour the mixture into a sample bottle and allow settling for 30minutes. Place the supernatant portion into a turbid meter and record your final value. Calculate the turbidity efficiency using equation 1. Repeat the following procedure using the settling time of 10- 30 minutes in 5.0 steps while keeping the other parameters constant.
Best Settling Time: Place 20ml of the coagulant dosage, 10ml of buffer 8 and 20ml of waste water into a beaker, using a turbid meter record your initial value. Stir rapidly for 4minutes, slowly stir for 20minutes at room temperature (29oC). Pour the mixture into a sample bottle and allow settling for 30minutes. Place the supernatant portion into a turbid meter and record your final value. Calculate the turbidity efficiency using equation 1. Repeat the following procedure using the settling time of 10- 30 minutes in 5.0 steps while keeping the other parameters constant.
Results and Discussions
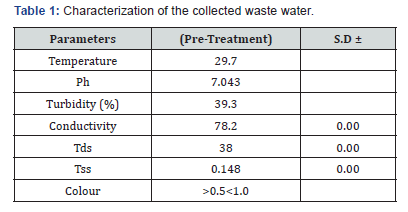
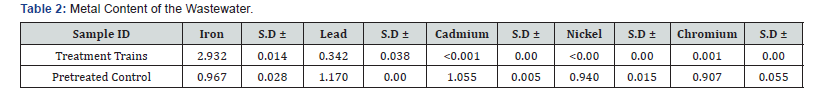
The proximate analysis of Banana peels has been studied [9]. The results of the characterization of the collected waste water are presented in (Tables 1 & 2). The pH of waste water was found to be 7.043 before treatment. This is similar to a reported work [10]. Also, the turbidity of the wastewater was found to be 38% with total dissolved solid of 38. Conductivity and TDS values were high in the water samples. This is possibly because the ions from the dissolved solids in the water samples created an ability of water to conduct an electrical current [10]. The metal content of the waste water was investigated using an (FAAS) atomic absorption spectrometer (Table 2). Result shows that there are high levels of metal content of lead, cadmium, chromium. The presence of these metals poses harmful effect to the aquatic environment.
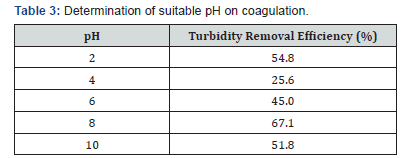
The highest turbidity removal efficiency was at pH 8 (Table 3). pH has a very important role to play on the ionic reactions too. This means that at pH 8, the coagulants cationic charged particles were equal to the contaminants or anionic suspension charged particles and thus destabilized them all by attracting them to coagulate or settle in the solution as suspension (Table 4).
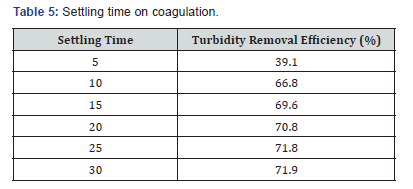
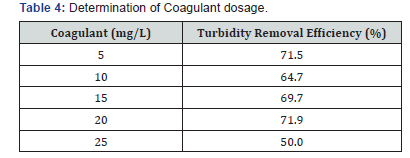
Table 4 represents the actual coagulant dosage that was needed. 20mg/L of the coagulant dosage gave the highest turbidity removal efficiency. The turbidity removal efficiency increased from 5 minutes to 30 minutes. However, the maximum was seen at 30 minutes (Table 5) with a turbidity removal efficiency of 71.9 %. This contrasts with a reported work [11].
Conclusion
The use of eco-friendly coagulant is really needed in the treatment of wastewater and paramount for the sustenance of human health, plant and aquatic life. Banana peel flocculants acts as a polymer body which continuously traps the dirt, forming more networks of monomer chains and causing itself and the dirt to fall to the bottom of the wastewater. It yielded a turbidity removal efficiency of 71.9%.
References
- Radin Maya Saphira Radin Mohamed, Amir Hashim bin mohd kassim, Adel Al-Gheethi (2017) Application of Natural Coagulants for Wastewater Treatment integrated water resources 1: 60-73.
- Sowmeyan R, Santhosh J, Latha R (2011) Effectiveness of herbs in community water treatment. Int Research J of Biochemistry and Bioinformatics 1(11): 297-303.
- Azizul R, Mohd-Suhaimi M, Othman N (2014) Biosorption of Pb(II) and Zn(II) inSynthetic Waste Water by Watermelon Rind (Citrullus lanatus). Appied Mechanics and Materials 465: 906-910.
- Nabilah Zayadi, Norzila Othman (2013) Removal of Zinc and Ferum Ions using Tilapia Mossambica Fish Scale. International Journal of Integrated Engineering 5(1): 23-29.
- Abdelaal A (2014) Eight International Water Technology Conference, Using a Natural Coagulant for Treating Wastewater. Faculty of Petroleum and Mining Engineering, Suez Canal University, Egypt, pp. 781-790.
- Sunita S, Sonal C (2014) Use of Tannin based natural coagulants for water treatment: An alternative to inorganic chemicals. International Journal of Chem Tech Research 6(7): 3628-3634.
- Sumathi T, Alagumuthu G (2014) Adsorption studies for arsenic removal using activated Moringa oleifera. Int J Chem Eng, p. 1-6.
- Saravanan J, Priyadharshini D, Soundammal A, Sudha G, Suriyakala K (2017) SSRG Wastewater Treatment using Natural Coagulants. International Journal of Civil Engineering 4(3): 40-42.
- Aakanksha D, Mane S (2013) Treatment of Industrial Wastewater by using Banana Peels and Fish Scales.
- Tan Chu Shan, Manaf Al Matar, Essam A Makky, Eman N Ali (2017) The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl Water Sci 7(3): 1369-1376.
- Vicky Kumar, Norzila Othman, Syazwani Asharuddin (2017) Applications of Natural Coagulants to Treat Wastewater-A Review MATEC Web of Conferences 103: 06016.






























