Comparative Studies of Groundwater in Akpugo Area of Enugu State, Nigeria
Nwanisobi Gloria*
Department of Chemical Engineering, Faculty of Engineering and Technology, Madonna University (Akpugo Campus) Enugu State, Nigeria
Submission: August 15, 2018; Published: September 10, 2018
*Corresponding author: Nwanisobi Gloria, Department of Chemical Engineering, Faculty of Engineering and Technology, Madonna University (Akpugo Campus) Enugu State, Nigeria, Email: glochinwa4real@yahoo.com
How to cite this article: Nwanisobi G. Comparative Studies of Groundwater in Akpugo Area of Enugu State, Nigeria. Organic & Medicinal Chem IJ. 2018; 7(5): 555724. DOI: 10.19080/OMCIJ.2018.07.555724
Abstract
The aim of this research work is to carry out a comparative study to assess the physicochemical parameters of groundwater within Akpugo area of Enugu state, Nigeria. Ten water samples were collected from ten communities within Akpugo area of Enugu State and analyzed for physicochemical characteristics. The heavy metals; iron, chromium, lead, zinc, silver, manganese, nickel and copper were analyzed using Atomic Absorption Spectrophotometer (AAS). The other parameters like chemical oxygen demand (COD) and biological oxygen demand (BOD) were also determined by various standard methods of water analysis. The BOD and COD profile varied from 3.8 to 3.6 and 128.040 to 356.232 respectively in the ten samples. The physicochemical parameters measured did not meet WHO standards for drinking water. Sanitary surveillance revealed that ten of the sampling points were littered with either animal or fowl dropping, or septic tanks/latrines were near the sampling points. The high concentrations of chemical oxygen demand and biological oxygen demand should raise serious public health awareness of over the quality of the community’s groundwater. Intervention measures including creating awareness and educating residents on borehole and hand-dug well construction, siting and care, boiling of water and improving sanitation should be urgently instituted and the State government should take necessary actions to treat the water to meet the WHO standard.
Keywords:Groundwater; Comparative Study; WHO Standard
Introduction
In the developing countries, water has been known to be indispensable resource for life quality. Availability of water has become a huge problem and has become a great concern to many families and communities [1]. Surface water bodies are not quite safe, they are prone to contamination due to natural alteration and anthropogenic intervention although in the long term, groundwater resources are reliable, safe, and accessible to people [2]. Water resources in Nigeria promote the standard of living and enhance economic growth. Like most places in the world, Nigeria is also experiencing population growth and demand for food and water is increasing too. In addition, there is climate change which impacts on the limited water resources in semi-arid regions [3]. Some authors have reported significant deterioration in microbiological quality of water between the source and the point of use in homes and that drinking water should be examined on physicochemical and microbiological quality [4]. According to literature, it has documented that there were estimated 4 billion cases of diarrhoea and 2.2 million cases of death annually recorded as a result of drinking unsafe water. Consequently, water borne diseases such as typhoid, cholera, diarrhoea and dysentery are possible due to this contamination [5]. The possibility of drinking unsafe water is well documented in many countries at all levels of economic development [6]. The most reliable source of drinking water has been reported to be bottled water which is good in bacteriological quality but is expensive [7]. Groundwater has become the major source of drinking water for people who do not have access to treated water. The need to access the quality of water from some of these alternative sources has become significant because they have direct effects on the health of individuals. In Akpugo, there is no periodic research on the quality (physicochemical) of boreholes and hand-dug wells which are a major source of drinking water. Hence, the need to conduct this research to provide health information about the state of these important sources of water in the community. Therefore, the aim is to determine the quality of ground water in Akpugo community by determining their physicochemical parameters.
Materials and Methods
Description of Study Area
Akpugo is found in Enugu state the Eastern part of Nigeria. Akpugo is Located in Nkanu West Local Government. It is also about 190miles (or 360km) south of Abuja, the country’s capital town. Akpugo, like every other Igbo clan, falls within the savannah region of the former Eastern Nigeria, usually characterized by tall trees and grasses (Figure 1). Five boreholes and five hand-dug wells were studied in this research. The boreholes and hand-dug wells were selected based on; existing conditions at the site, properties of the soil and anthropogenic activities at the site (Figure 2).
Sampling and Sample Treatment
In order to avoid microbial contamination of sample containers, fresh bottled water (500ml) was bought and carefully emptied by preventing any contamination from handling at the sampling site prior to sampling. New fresh bottles were used for subsequent sampling. 1.5-liter plastic bottles were rinsed with distilled water before using them for sampling. Sampling bottles were labeled A-J to represent the ten communities respectively. Ice-box was used to convey the samples which were rinsed with distilled water and ice packs kept inside to preserve the sample before taking to the laboratory for analysis.
Sample Analysis
Preparation of Mixed Metal Standard Solution: 100ppm stock solution was prepared by weighting 1g equivalent of each metal salt and dissolving in deionized water. Solutions of zinc, iron and copper were transferred quantitatively into a 1-liter standard flask and acidified by adding 10ml conc. HNO3 before diluted to the mark with deionized water. The lead solution was prepared in a separate 1-liter standard flask in the same manner. All gave 1mg/ml stock solution of the metals. From these, 10mg/l solution were prepared by diluting 10ml of the solution to 1 liter. Mixed standard solutions of zinc and copper 0.3, 0.6, 1, 1.5 and 2ppm each and 0.6, 1.2,2,3 and 4ppm of lead were prepared by taking appropriate volumes of the 10ppm solution into 1-liter standard flask and diluting to the mark with deionized water.
Determination of the Concentration: The hollow cathode lamp for each element to be analyzed was selected as well as the wavelength. After zeroing the instrument with the blank, the serially diluted standard solution of each element was aspirated, and their absorbance recorded. A calibration curve was plotted using the standard absorbance against concentration for which the concentration of the elements in the samples were obtained by extrapolation.
Biological Oxygen Demand (BOD): Add 1.9cm3 of solution A to the sample (250cm3 in the sample bottle filled nearly to the brim) and 1.0cm3 of solution B, using a pipette. Stopper the sample and shake thoroughly, inverting several times. Allow to settle, to observe white precipitation of Mn (OH)3. Then add 1.5cm3 of concentrated sulphuric acid, and restopper the bottle and mix thoroughly to dissolve the precipitate. Then with a pipette, withdraw 25cm3 of the sample into a titrating flask, titrate with standard 0.1M Na2S2O3 to nearly faint yellow solution. Then add the starch indicator and continue adding the titrant until the blue solution turns like original water sample. Record the Volume and repeat the process for the same volume of water kept for 5 days at the same time.
Results and Discussions
In this study from (Table 1), iron content varied from 0.002 to 0.027mg/l in samples (A, B, D, G, H, I) and there was no trace of iron found in sample (C, E, F, J). Low iron concentration was recorded in some of the sample while the remaining 40% recorded no trace of iron. The lowest value obtained was 0.002 in sample G which is way below the 1.0mg/l permissible level recommended [8] in drinking water. Although, iron is an important dietary requirement in humans needed by hemoglobin and good for several other functions, when high concentrations of iron are absorbed, iron is stored in the pancreas, the liver, the spleen and the heart. This may damage those vital organs. Chromium is widely distributed in the earth’s crust. It can exist in the oxidation states of +2 and +6 soils and rocks may contain small amount of chromium, almost always in the trivalent state. In general, the chromium concentration in groundwater is low (0.007mg/l). The world health organization recommended maximum allowable concentration in drinking water of chromium as 0.05 milligram. ln this study 40% of the samples (D, F, G, I) had no trace of chromium in them, the remaining 60% of the samples (A, B, C, E, H, J) have chromium concentration above the limit of 0.05mg/ l set standard by WHO [9]. In this study, maximum level of lead concentration (0.1637, 0.1637, 0.3275) were found in samples (B,G,I) respectively while the other samples (A,C,D,C,E,F,H,J) had no trace of lead. The concentration of lead in samples (B, G, I) are at high levels. Compared to samples B and G, sample I has the highest level of lead concentration.
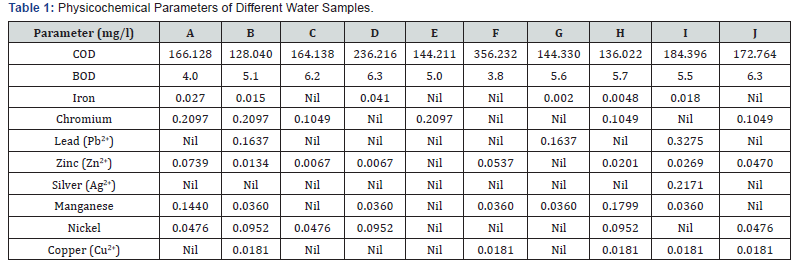
The concentrations of lead in these samples are above the 0.01mg/l limit set by WHO. Zinc is one of the important trace elements that play a vital role in the physiological and metabolically process of many organisms. Nevertheless, higher concentration of zinc can be toxic to the organism [10]. It plays an important role in protein synthesis and is a metal which shows low concentration in surface water due to its restricted mobility from the place of rock weathering or from the natural sources [11]. In this study a minimum of 0.0067 mg/l was recorded for samples C and D and maximum concentration of 0.0739 and 0.0537 mg/l were recorded for samples C and F respectively and samples (B, H, I, J) had concentrations ranging from 0.01 to 0.047 mg/l, samples E and G had no trace of zinc. The concentration of zinc in all samples falls below the WHO limits of 5.0 mg/l which is the highest desirable level for Zinc in drinking water. Silver is a valuable, naturally occurring, metallic element. Silver was absent in the nine samples but present in sample I, with concentration of 0.2171mg/l which is above the limit of 0.0001mg/l set by the world health organization (WHO).
The aesthetic objective for manganese in drinking water is 0.05mg/l. The concentration of the manganese ranged from 0.144 to 0.0360 in 70% of the sample (A, B, D, F, G, H, I) while in the other 30% of the samples had no trace of manganese (C, E, J). The concentration of manganese in all the samples fall below the WHO limits with concentrations 0.1440 mg/l and 0.1799 mg/l respectively. Four of the samples collected had no traces of nickel but the six samples had traces of nickel above detectable limit of 0.02mg/l. Contamination of drinking water with high level of copper may lead to chronic anemia [12]. Copper in excess could impart a bitter taste of water and promotes the corrosion of galvanized iron and steel fitting [13]. 50% of the samples (A, C, D, E, G) had no traces of copper and the other 50% of sample (B, F, H, I, J) contained 0.0181 mg/l each of copper, the concentration of copper is also below the 1.0mg/l set standard. This indicates that copper is found in a considerable or safe amount in the samples.
Conclusion
The water samples analyzed in this study are not suitable for consumption in terms of physiochemical quality since the tested parameters did not meet the WHO guideline values for drinking water. On the other hand, there were high bacteriological indicator counts in them due to COD and BOD results gotten, they were extremely above WHO recommended guideline values for drinking water. Thus, the groundwater (well) analyzed are not wholesome for consumption with respect to physiochemical parameters determined.
References
- Okonko IO, Ogunjobi AA, Adejoye AD, Ogunnusi TA, Olasogba MC (2008) Comparative Studies and Microbial Risk Assessment of Different water Samples used for processing Frozen sea Foods in Ijora-Olopa, Lagos State, Nigeria 7: 16.
- Mygatt E (2006) World’s Water Resources Face Mounting Pressure. Earth Policy Institute. Washington, DC, US, p. 4.
- Herrera Pantoja M, Hiscock KM (2008) The Effects of Climate Change on Potential Groundwater Recharge in Great Britain. Hydrological Processes 22(1): 73-86.
- Chant CL, Zalifah MK, Narrakiah AS (2007) Microbiological and Physicochemical Quality of Drinking Water. Malaysian Journal of Analytical Science 11(2): 414-420.
- Musa HA, Shears P, Kafi S, Elsabag SK (1999) Water quality and public health in Northern Sudan: A study of rural and peri-urban communities. J Appl Microbiol 87(5): 676-682.
- Dufour A, Snozzi M, Koster W, Bartram J, Ronchi E, et al. (2003) Assessing Microbial Safety of Drinking Water. Improving Approaches and Methods, OECD, p. 11.
- Obiri Danso, Okore Hanson, Jones K (2003) The Microbiological quality of drinking water sold on the streets in kumasi Ghana. Lett Appl Microbiol 37(4): 334-349.
- World health organization (1998) World health organization’s guidelines for drinking water. Geneva, p. 1.
- Nkono NA, Asubiojo OI, Ogunsua AO, Oluwole AF, Wardi NI, et al. (1997) Trace Elements in Drinking and Groundwater Samples in Southern Nigeria. The Science of the Total Environment 208(1-2): 1-8.
- Rajkovic MB (2008) Identification of metals in drinking water by indirect analysis method based on scale tests. Sensors 8: 2188-2207.
- Rajappa B, Manjappa S, Putttaiah E (2010) Monitoring of Heavy Metal Concentration in Groundwater of Hakinaka Taluk, lndia. Contemporary Engineering Sciences 3(4): 183-190.
- Acharya GD Hathi MV, Patel AD, Parmar KC (2008) Chemical properties of groundwater in Bhiloda Talukka Region, North Gujarat, lndia. E-journal of Chemistry 5(4): 792-796.






























