Antioxidant and Free Radical Scavenging Properties of Seed Components of Pongamia Pinnata -A Comparative Study
Arpita Ghosh Mitra1*, Suvra Mandal2, Priyabrata Das3, Swati Dasgupta3, Soma Mukhopadhyay3, Asish Mukhopadhyay3, Julie Banerjee4 and Manoj Kar5
1Hla & Molecular Lab, Medical Super Specialty Hospital, India
2Department of Chemistry, Central Research Institute (Ay), India
3Department of Biochemistry & Molecular Biology, Netaji Subhas Chandra Bose Cancer Research Institute, India
4Department of Chemistry, University College of Science, India
5Department of Biochemistry, NRS Medical College & Hospital, India
Submission: August 11, 2018; Published: September 10, 2018
*Corresponding author: Arpita Ghosh Mitra, HLA & Molecular Lab, Medical Super Specialty Hospital, India, Email: arpitaghoshmit@gmail.com
How to cite this article: Arpita G M, Suvra M, Priyabrata D, Swati D, Soma M, et al. Antioxidant and Free Radical Scavenging Properties of Seed Components of Pongamia pinnata -A Comparative Study. Organic & Medicinal Chem IJ. 2018; 7(5): 555723. DOI: 10.19080/OMCIJ.2018.07.555723
Abstract
Background: According to recent periodicals different parts of Pongamia pinnata, a well-known medicinal plant from Indian Ayurveda literature has potent antioxidant activities.
Aims: Our aim is to study is to reveal the antioxidant properties of active compounds of the seed extract from Pongamia pinnata seed oil.
Methods: Active ingredients, Karanjin and Pongapin and Yellow oil are responsible for medicinal activities were isolated from hexane extract of the seed oil. Nitric oxide and superoxide quenching properties, total antioxidant status and iron chelating activities were estimated by standard spectrophotometric protocol.
Results: The active compounds from seed extract oil show variable nitric oxide, superoxide and iron chelating activities associated with potent total antioxidant status. The IC50 values of karanjin and pongapin were 7.1 ± 0.6 μM and 9.8 ± 0.2 μM respectively.
Conclusion: The active compounds isolated from Pongamia pinnata seed extracts have potent total antioxidant activity, superoxide and nitric oxide scavenging activity, iron chelating activity.
Keywords:Pongamia pinnata; Karanjin; Pongapin; Nitric Oxide Scavenging Activity; Superoxide Scavenging Activity; Total Antioxidant Status; Iron Chelating Activity
Introduction
Pongamia pinnata Pierre syn. Pongamia glabra Vent. (Fabaceae) popularly known as Karanja is a medium sized glabrous tree with short bole and spreading crown. Seeds 1-2, elliptic or wrinkled, 1.7-2.0cm long and 1.2-1.8 cm broad with reddish brown leathery test [1]. The plant is found throughout India and further distributed eastwards, mainly the littoral region of south eastern Asia and Australia. In the ayurvedic literature of India different parts of this plant have been recommended as a remedy for various ailments and have been used in traditional medicines for rheumatic joints [2] diabetes [3] and sores [4]. Different extracts of leaves have been reported to have anti-inflammatory activity [5]. Leaf extract also have found to be antihyperammonemic effect [1]. The seed oil is known to have value in folk medicine in human and animal skin diseases. It is effective in enhancing the pigmentation of skin leucoderma or scabies [2,4]. Recent work suggests that leaf extract of Pongamia pinnata has a strong antioxidant and anti-lipid peroxidation property in ammonium chloride induced hyperammonemia rats. Although its seed extract was suggested to be an important medicine in ayurvedic literature. The seeds of Pongamia pinnata contains huge amount of oil. Fractions contain various flavonoid compounds, fatty acids, flavone, glycosides, chalcones and reviews have emphasized that these compounds possess numerous biological and pharmacological properties and has potential therapeutic interest [6]. Thus, the objective of the present study is to isolate different constituents of the hexane extract of the seeds of Pongamia pinnata which contains various flavonoids. The study was followed by its structure determination along with estimation their total antioxidant status compared to Trolox (TAS), iron chelating activity, superoxide radical quenching activity and scavenging activities against nitric oxide [7].
Materials and Methods
Chemicals: Oxidized cytochrome c, napthylethelenediamide were purchased from Sigma chemicals, ABTS (3-ethylebenzothiazoline-6-sulfonic acid diammonium salt) from Aldrich, DMSO (dimethyle sulfoxide) from SRL, India, Rest chemicals were all AR grade and purchased from BDH [8,9].
Plant Material
Chemical Investigation: Pongamia pinnata seeds were collected from local supplier of Kolkata and authenticated by Dr. S.R Das, Ex-Survey officer, Central Research Institute (Ayurveda), Kolkata, India.
Isolation and Identification of the Chemical Constituents of the Seeds of Pongamia pinnata: Air dried and powdered matured seeds (2kg) were extracted in hexane in a soxhlet apparatus for 48hrs. The solvent was removed from the extract and the oily residue was collected. It was kept in refrigerator for cooling when a waxy solid separated out [10]. The solid was filtered and chromatographed over silica gel (60-120mesh) column hexane-ethyl acetate (9:1) eluents afforded a white low melting solid designated as Karan (H) 1. IR spectrum revealed that it was a fatty acid. The filtrate was again kept in refrigerator for cooling when another pale-yellow solid separated out. It was filtered and purified by repeated crystallization with hexane - acetone mixture. A white shining crystalline solid, Karan (H) 2, Rf 0.29 (hexane -acetone 3:1), mp. 158-160ºC was obtained. Finally, the residual large amount of yellow oil (~300ml) was chromatographed over silica gel (60-120mesh) column using solvent and their mixture with increasing polarity. First few hexane eluents afforded a yellow colored oil fraction and next hexane eluents on concentration yielded orange oil. Both the oil was mixture of fatty acids. The column was then eluted with hexane-acetone (9:1) mixture and two solid compounds were obtained, one of which was previously isolated Karan (H) 2 and second compound was isolated as a pale-yellow solid [11]. It was crystallized from acetone to get a pure crystalline compound, Karan (H) 3 having Rf 0.32 (hexane – acetone 3:1 mixture), mp. 190- 191ºC. Karan (H) 2 was identified as a furano flavone derivative, Karanjin. All chemical and spectroscopic data are in accordance with these reported in this literature. Karan (H) 3 was characterized as another furano flavone derivative, pongapin by detailed spectroscopical (UV, IR, PMR, CMR and Mass) analysis. Karanjin and pongapin, these two furanoflavonoids were isolated previously from the same source but detail spectral studies for the characterization of the compounds have been done including carbon magnetic resonance spectroscopy, which are being reported for the first time [12,13].
Biochemical Investigation
Materials:
a) Oxidized cytochrome c, napthylethelenediamide were purchased from Sigma chemicals.
b) ABTS (3-ethylebenzothiazoline-6-sulfonic acid diammonium salt) from Aldrich.
c) DMSO (dimethyle sulfoxide) from SRL, India.
d) Rest chemicals were all AR grade and purchased from BDH.
Methods:
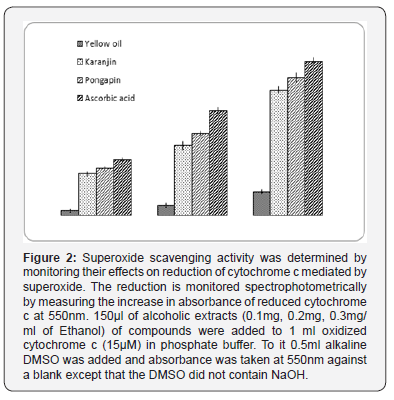
a. Scavenging Activity against Super Oxide Anion: Superoxide scavenging activity was determined by monitoring their effects on reduction of cytochrome c mediated by superoxide [13]. The reduction is monitored spectrophotometrically by measuring the increase in absorbance of reduced cytochrome c at 550nm. 150μl of alcoholic extracts (1mg/ml) of compounds were added to 1ml oxidized cytochrome c (15μM) in phosphate buffer. To it 0.5ml alkaline DMSO was added and absorbance was taken at 550nm against a blank except that the DMSO did not contain NaOH. A control was made without addition of the extract. A stable solution of superoxide is prepared by adding 1ml 0.5M NaOH in 100ml DMSO and incubating it 30min at room temperature. 15μM oxidized cytocrome c is prepared in 200mM phosphate buffer pH 8.6containing 100μM EDTA. Percentage of the superoxide scavenging activity was estimated from difference of sample reading from control.
b. Scavenging Activity against Nitric Oxide: A modified method of scavenging effect against the nitric oxide was estimated according to the method of Lucia Marcocci et al. [14]. In brief 0.5ml of 10mM sodium nitroprusside solution and 0.5ml of ethanolic extracts (0.3mg/ml) of the compounds isolated from Pongamia pinnata seed were incubated 150min at ambient temperature. At the end of the incubation 1ml Griess reagent (Griess reagent is prepared by mixing equal volume of 2% sulphanilamide in 4% phosphoric acid and 0.2% naphthyle ethelenediamide) was added and nitric oxide level was estimated spectro photometrically at 542nm. Percentage of inhibition of nitric oxide of each compound was estimated from the difference of absorbance from control and test. Nitric oxide quenching activity was calculated from the differences of absorbance of control.
The control absorbance – The test absorbance x 100 = % of Nitric oxide inhibition Control absorbance
c. Total Antioxidant Status: Total antioxidant status was estimated according to the method of Re R et al. [15] based on the inhibition of radical cation ABTS which has a characteristic long wavelength absorbance maximum at 734nm. ABTS radical cation is formed by interaction with potassium per sulfate. The total antioxidant status value is defined as the mM concentration of Trolox solution having the same antioxidant capacity as a 1mM solution of substrate. Ethanol does not act as antioxidant value which therefore we use to solubilize substances in our assay. Methods of measuring TAS was based on inhibition of the absorbance of the radical cation ABTS+ which has a characteristic absorbance spectrum at 734nm by antioxidant present in plant components dissolve in ethanol (0.3mg/ml) against a known standard antioxidant Trolox (1mM).
d. Iron Chelating Activity: Deoxyribose assay for detecting hydroxyl radicals. This was estimated by the method of Okezie I Aruoma [16]. In performing the assay, the reaction mixture contain the final volume of 1.2ml, the following reagents and the final concentrations, deoxyribose (2.8mM), ferric chloride (25 mM), EDTA (100μM) are premixed. Hydrogen peroxide (2.8mM), phosphate buffer (10mM) at pH 7.4, compounds to be tested at a concentration upto 20mM and ascorbate (100μM). Ascorbate is added to start the reaction and incubate at 37˚C for 1hr. 1ml of 1% (w/v) TBA in 50mM NaOH and 1ml of 2.8 % (w/v) TCA are added to the reaction mixture which then heated 30mins in boiling water bath to develop colour of MDA like product to develop deoxyribose damage. The resulting pink colour is extracted after cooling in 2ml butanol and absorbance is taken at 532nm.
e. Statistical Analysis: All experiments were done in triplicate. Statistical calculation has been done accordingly and all the values were expressed as mean ± SEM (Standard Error of Mean).
Statistical analysis of plant compounds using SPSS12. Statistical evaluation was calculated by 2 tail tests. For all comparisons, p< 0.05 was considered as significant.
Results
Scavenging Activity Against Nitric Oxide
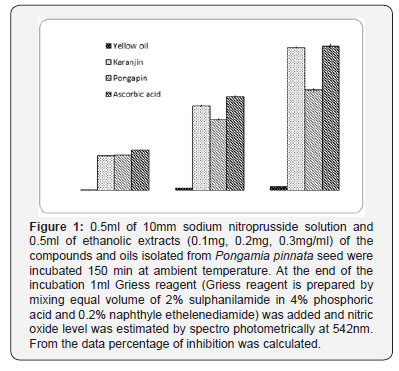
Nitric Oxide (NO) scavenging assay is based on the scavenging ability of the seed oil components as well as ascorbic acid, which is used as standard. The scavenging of NO was found to increase in dose dependent manner. Maximum inhibition of NO was observed in the components of highest concentration (0.3mg/ml of Ethanol) for both the samples. At this maximum concentration, inhibition was found to be 89.14% for ascorbic acid, which serves as the standard. For seed extract extract components, inhibition was found to be higher 88.47% in Karanjin, and similarly it is 62.31% and 2.4% in Pongapin and Yellow oil (Figure 1).
Scavenging Activity Against Super Oxide Anion
Percentage scavenging of superoxide anion examined at different concentrations of Ethanol extract of pongamia pinata (0.1mg,0.2mg,0.3mg/ml) was depicted in Figure 2. The percentage scavenging of superoxide radical surged with the enhanced concentration of plant extract. The maximum scavenging activity of Pongapin (37.9%) compared with the other extracted purified compound Karanjin (34.47%) and Yellow oil (6.47%) respectively. Superoxide scavenging ability of plant extract might primarily be due to the presence of flavonoids.
Total Antioxidant Status
The antioxidant capacities of the Yellow oil, Karanjin and Pongapin isolated purified compounds from Pongamia pinnata were evaluated by measuring their ability to scavenge ABTS radicals. ABTS has been extensively used as a free radical to evaluate antioxidant substances that reduce ABTS by donating hydrogen to form the non-radical ABTS+. The Yellow oil, Karanjin and Pongapin showed ABTS radical scavenging at 0.3ul. The highest ABTS scavenging capacity was observed in the Pongapin (0.61%), which is greater than another purified compound Karanjin (0.25%) and Yellow oil (0.57%).
Iron Chelating Activity: Deoxyribose Assay for Detecting Hydroxyl Radicals
The formation of OH radical by the Fenton reaction is representative of the events that occur in vivo in iron-rich tissues like liver, where it contributes to the initiation of lipid peroxidation (Porter 1984). Therefore, the scavenging activity of this radical can be considered as one of the best indicators of the antioxidant potential of a compound. In this assay the Yellow oil, Karanjin and Pongapin showed their capability to protect oxidative degradation of the deoxyribose sugar at dose dependent manner. The percentage of the inhibition activity was more in pongapin (87.12%) than the Karanjin (30.17%) and the yellow oil (81.27%). The levels of hydroxyl radical scavenging activity of components were also like those of the ascorbic positive control [17].
Discussion
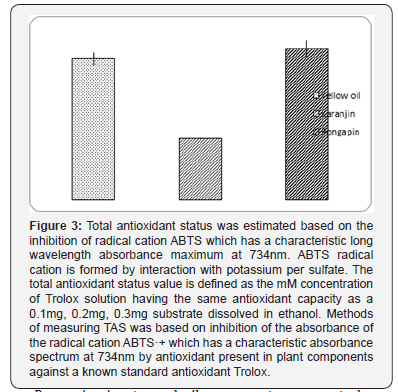
Pongamia pinnata seed oil components appears to be an efficient multifunctional antioxidant compounds for protection against oxidative stress mediated injuries. Nitric oxide and superoxide quenching activity and strong antioxidant status may be among the important factors responsible for the medicinal value of a compound. It is thus warranted that new drug can be formulated from active constituents of Pongamia pinnata seed oil for ailment of the iron overloaded diseases or NO mediated disorder (Psoriasis, SLE, Rheumatoid arthritis, leukemia, Cancer, Crohn’s) where nitric oxide plays the key role to controlling those disease. It has been found from present study that Karanjin has shown nitric oxide scavenging activity as same as ascorbic acid and pongapin also has a remarkable (lower than ascorbic acid) scavenging activity [18]. Whereas yellow oil did not show any scavenging activity. In case of Total antioxidant status (TAS) compared to Trolox appeared as shown in Figure 3. to be very high in compounds associated with nitric oxide and super oxide quenching properties indicating a synergistic activity. Compounds also show iron chelating activity (Figure 4) which might be an important factor for disease condition where non-heme iron mediated hydroxyl radical generation by Fenton reaction causes cellular insult viz. in diabetes mellitus, thalassemia and psoriasis [19-21].
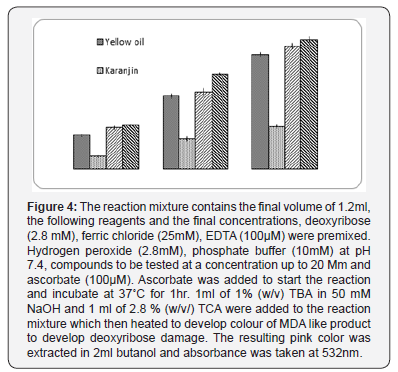
It is difficult to define the biological consequences of the interaction of the seed oil with nitric oxide considered the multifunctional role of this radical [22]. Nitric oxide identified as being produced in different types of the mammalian cells by different isoenzymes of nitric oxide syntheses (NOS). Endothelial NOS and neural NOS are constitutive in nature and provide continuous low level of production of nitric oxide whereas inducible NOS usually generated for immune defense mechanism and formed by the stimulation of the cytokines mainly from activated macrophages which have an anti-bactericidal and cytotoxic effect [23]. Excessive formation of nitric oxide plays a pivotal role in cytotoxic activity in several immunological diseases viz. Rheumatoid arthritis, SLE, Crohn’s and Psoriasis. Among the cells of immune system macrophages are the principal producers of nitric oxide. In psoriasis accumulated nitric oxide is reported to induce rapid proliferation, differentiation and inhibition of apoptosis of keratinocyte cell in the epidermis which might be responsible to form psoriatic plaque. Furthermore, nitric oxide reacts with superoxide radical to form peroxy nitrite anion which can initiate lipid peroxidation in psoriatic tissue plaque. Moreover, interaction of nitric oxide with ferritin, a reservoir of iron pool in the system resulting in free iron release and consequence of formation of toxic hydroxyl radical (OH) in the presence of hydrogen peroxide by Fenton reaction [9]. Thus, iron chelating property of our drug associated with nitric oxide and superoxide quenching properties of Karanjin and Pongapin may help to overcome the secondary complicacies of different iron overload diseases. Figure 4 show Karanjin and Pongapin have strong iron chelating activity. Possibly we first reported these noble properties like superoxide and nitric oxide quenching activities of purified compounds of Pongamia pinnata seed extract which may responsible for the medicinal properties against various diseases.
References
- Essa MM, Subramanian P, Suthakar G, Manivasagam T, Dakshayani KB (2005) Protective influence of Pongamia pinnata (Karanja) on blood ammonia and urea levels in ammonium chloride induced hyperammonemia: anti hyperammonemic effect of the leaf extract. J Appl Biomed 3(3).
- Chopade VV, Tankar AN, Pande VV, Tekade AR, Gowekar NM, et al. (2008) Pongamia pinnata: phytochemical constituents, traditional uses and pharmacological properties: A review. International Journal of Green Pharmacy 2: 72-75
- Punitha R, Manoharan S (2006) Anti-hyperglycemic and anti-lipidperoxidative effects of Pongamia pinnata (Linn.) Pierre flowers in alloxan induced diabetic rats. J Ethnopharmacol 105(1-2): 39-46.
- Chatterji A, Pakrashi SC (1992) Pongamia pinnata. In The treatise on Indian Medicinal Plants 2: 110-112.
- Srinivasan K, Murugananda S, Lal J, Chandra S, Tandan SK, et al. (2001) Evaluation of anti-inflammatory activity of Pongamia pinnata Pierre leaves in rats. J Ethnopharmacol 78(2-3): 151-157.
- Hoult JRS, Moroney MA, Paya M (1994) Actions of Flavonoids and Coumarins on Lipoxygenase and Cyclooxygenase. Methods Enzymol 234: 443-454.
- Villar A, Gasco MA, Alcaraz MJ (1994) Anti-inflammatory and anti-ulcer properties of hypolaetin-β-glucoside, a novel plant flavonoid. J Pharm Pharmacol 36(12): 820-823.
- Otsuka H, Fujioka S, Komeya T, Mizuta E, Takamoto M (1982) Studies on anti-inflammatory agents. Part 6. Anti-inflammatory constituents of Cinnamomum sieboldii Meissn. Yakugaku Zasshi 102(2): 162-172
- Reif DW, Simmons RD (1990) Nitric oxide mediates iron release from ferritin. Arch Biochem Biophys 283(2): 537-541.
- Ormerod AD, Weller R, Copeland P, Benzamin N, Ralston SH, et al. (1998) Detection of nitric oxide and nitric oxide synthases in psoriasis. Archives Dermatol Res 290(1-2): 3-8.
- Aneja R, Khanna RN, Seshadri TR (1963) 6-Methoxyfuroflavone, a new component of the seeds of Pongamia glabra. J Chem Soc 163.
- Garg GP, Sharma NN, Khanna RN (1978) Two new fuano compounds, glabra-I and glabra-II from the stem-bark of Pongamia glabra. Ind J Chem 16(8): 658-661.
- Marcocci L, Packer L, Marie Therese Droy Lefaix, Sekaki A, Albert MG (1994) Antioxidant action of Ginkgo biloba extract EVb. Methods Enzymol 234: 462-475.
- Marcocci L, Suzuki YJ, Tsuchiya M, Packer L (1994) Antioxidant activity of nitecapone and its analog OR-1246: Effect of structural modification on antioxidant action. Methods Enzymol 234: 462-475.
- Re R, Pellegrini N, Proteggenate A, Pannala A, Young M, et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9-10): 1231-1237.
- Aruoma OI (1994) Deoxyribose assay for detecting hydroxyl radicals. Methods Enzymol 233: 57-66.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, et al. (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126(1): 131-138.
- Gokhale NR, Belgaumkar VA, Pandit DP, Deshpande S, Damle DK (2005) A study of serum nitric oxide levels in psoriasis. J Dermatol Venereol Leprol 71(3): 175-178.
- Ghosh A, Mukhopadhyay S, Kar M (2008) Role of free reactive iron in psoriasis. Indian J Dermatol Venereol Leprol 74(3): 277-278.
- Kar M, Chakraborty AS (1999) Release of iron from hemoglobin-a possible sourse of free radicals in diabetes mellitus. Ind J Exp Biol 37(2): 190-192.
- Chakraborty I, Mitra S, Gachhui R, Kar M (2009) Non-heme iron mediated oxidative stress in hemoglobin E-beta Thalassemia. Annal Acad Med 39(1): 13-16.
- Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: Physiology, pathophysiology and pharmacology. Pharmacol Rev 48: 109-142.
- Christian B (2001) Nitric oxide and the immune response. Nature Immunology 2(10): 907-916.






























