Vacuum Liquid Chromatography: Simple, Efficient and Versatile Separation
Anupam Maurya1*, Komal Kalani2, Subash Chandra Verma1, Ravinder Singh1 and Anupam Srivastava3
1 Pharmacopoeia Commission for Indian Medicine and Homoeopathy, Ministry of AYUSH, Ghaziabad, India
2 Department of Pharmacology and Toxicology, Higuchi Bioscience Centre, University of Kansas, USA
3 Ministry of AYUSH, Govt. of India, India
Submission: June 11, 2018; Published: June 15, 2018
*Corresponding author: Anupam Maurya, Pharmacopoeia Commission for Indian Medicine and Homoeopathy, Ministry of AYUSH, Govt. of India, Ghaziabad, India 201002, Tel:+91-7505654582 ; Email: manupamk@gmail.com
How to cite this article: Anupam M, Komal K, Subash C V, Ravinder S, Anupam S K. Vacuum Liquid Chromatography: Simple, Efficient and Versatile Separation Technique for Natural Products. Organic & Medicinal Chem IJ. 2018; 7(2): 555710. DOI: 10.19080/OMCIJ.2018.07.555710
Opinion
Almost 50% of drugs directly or indirectly derived by Natural product, which is used for human welfare. The natural products (phytomolecules) exist in plant matrix as a very complex mixture, from which the product of interest isolated and purified. For the isolation of natural products from plant sources, the good selection of appropriate techniques and approaches are essential. The importance of proper isolation and purification techniques is well recognized for medicinal and aromatic plants. Among various techniques for separation of natural products, the chromatographic techniques, which are fast, rapid and widely used in separation. For these reasons and also with the aim of finding simple solutions to the complex separation problems, a number of new chromatographic methods, both analytical and preparative have been developed. Most of the separations are carried out in two steps. In first step a crude separation is achieved with a high load on cheap stationary phases. Traditionally for this purpose liquid-liquid extraction and fractionation is carried out first, which is then followed by liquid–solid isolation chromatographic separation (planar & column chromatography) over silica gel or alumina etc. Among the all chromatographic techniques, Vacuum Liquid chromatography (VLC) is most efficient in both crude as well fine separations of the complex synthetic and natural products mixture. In the last decade VLC has been progressively applied in the field of natural products as well as in synthetic chemistry because of its simplicity of operation [1].
Vacuum Liquid Chromatography (VLC)
VLC was first used for the separation of diterpenes in Australia. In 1997 a short description of the method for isolation of diterpene from an Australian soft coral was published, but no apparatus and method details were reported. The name Vacuum Liquid chromatography (VLC) was first coined by Targett et al. [2]. VLC is considered as a preparative thin layer chromatography (PTLC) as separation is carried out on TLC grade silica gel or aluminium oxide and column is dried after each fraction as in PTLC plates are dried and re run to enhance the separation. The packed VLC columns can be reused for the same or similar separation thoroughly washing the column with methanol and scrapping away the decomposed polar material or band from the top of the adsorbent column. Gradient elution is very effective and can be used for the separation of small as well as large amount of mixtures. Various applications are available for separation and isolation of diterpenes, isobenzofuranone, triterpens glycoside, limonoids, xanthanoes, iridoids, flavonoids glycosides and alkaloids [3].
Apparatus
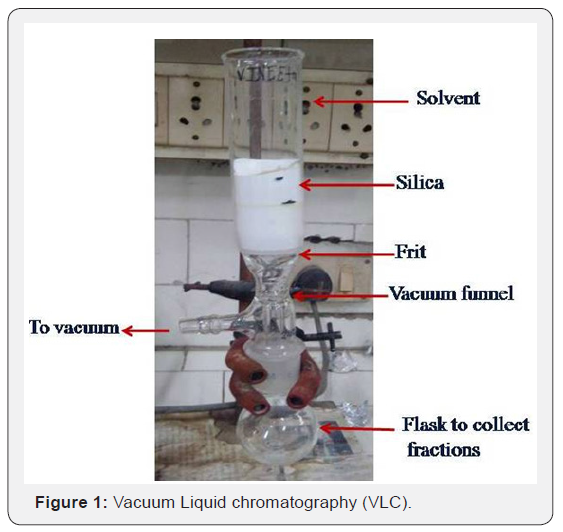
There is no special requirement for a VLC apparatus (Fig.1). It can be easily made with the available glassware in the laboratory. It requires a sintered glass funnel with fritted disk grade G3 (15-40μ) or G2 (40-90μ), G1 (90-150). A laboratory water pump or water aspirator to provide 20-70 mmHg vacuum is required for suctioning. The apparatus is set up as shown in Figure 1.
Sample and Column Size
For various scale separations, sample amount and column size are given below:
Further large-scale separations can be carried out on 250, 500 and 1000 ml sintered funnels. For more convenient application height of the column can also be increased as per the user requirement by getting fused a similar diameter glass tube [4].
Adsorbents and operations
In VLC, generally TLC grade binder free silica gel (silica gel H) or aluminium oxide are used, but the other adsorbents can also be tried on the basis of their TLC profile. Packing of VLC, column is easy and simple. The dry adsorbent is loaded into the sintered funnel and first allowed to settle under gravity with gentle tapping of the column with a rubber tube as in case of Column Chromatography (CC). The layer can be first pressed with glass or rubber stopper. Once the adsorbent is settled, vacuum should be applied and adsorbent layer should be further pressed with stopper to finally pack a uniform and tight column. In order to check the uniformity of the column, vacuum should be released and quickly a non-polar solvent (such as hexane) should be poured on the top of the adsorbent and vacuum applied. The solvent must pass uniformly through the column otherwise it has to be repacked. Before sample loading, column should be dried under vacuum [4].
Loading of Sample
In VLC, liquid loading of the sample is preferred as it is easy and fast. However solid loading in the form of slurry can also be done. If the loading sample is liquid, it can be loaded as such otherwise in case of solids, sample should be dissolved in the least polar solvent. The properly dissolved sample should be introduced on the surface of adsorbent with the help of a dropper. It must be noted that during sample loading the column should be without vacuum. Once the total sample has been introduced, then vacuum should be applied. In this way sample is loaded as a uniform nice band on adsorbent. The smaller the band, the better will be separation. The column is left under vacuum for 5-10min to take out the polar solvent used to dissolve the sample. The column is then run with the suitable solvent mixtures under vacuum. A moderate vacuum from 20-70mmHg should be used to prevent the loss of solvent due to evaporation. Fractions can be collected in standard joint round bottom flasks. After each fraction solvent is added to the top of the column and then vacuum applied. In order to stop channelling, it is advisable to suck the column dry after each fraction [2,4,5].
Column Elution
Although gradient elution of the column is preferred but isocratic elution can also be carried out. Loss of volatile solvents can be minimized by using a large pore size sintered glass funnel or by increasing the flow rate.
Applications
Purified Natural products are required for the drug development and supply of authentic pharmaceutical standards for synthetic and analytical work. The VLC was successfully applied for isolation and purification of natural products such as hopeaphenol (1) and α-viniferin (2) from Shorea ovalis, Beta-sitosterol (3) from the Bark of Sarcocephalus latifolius and rotenone (4) from Derris elliptica. VLC was also used in concentration of palm carotene from crude palm oil [1,6,7] (Figure 2).
Epilogue
As all-natural compounds exist as very complex mixture in matrix, there is constant need in organic chemistry to separate both large and small quantities of mixture efficiently rapidly and inexpensively. We have successfully applied VLC for the fractionation and isolation of plant as well synthetic molecules The typical sample and solid support ratios used in VLC are from 30:1 to 300:1, according to the number of constituents and their complexity on TLC profile, user can choose a suitable ratio as resolution can be improved by increasing the sample to support ratio. As per observation, VLC is superior to PTLC and Column Chromatography (CC), mainly in the bioactivity activity guided extraction, fractionation and isolation of natural products. Unlike CC, stationary phase in VLC can dry and sample can be loaded direct without preparing slurry, after washing the VLC column with methanol (10%), it can be reused. The excellent separation on VLC is due to the fine particle size (10 micro meter average) which provides a very large number of theoretical plates. The separation is economical in terms of support, solvents, and in time of carrying the experiment. It is very useful in both large and small quantities of mixture as well as well crude and fine separation. There is various type of VLC apparatus according to applications are easily available in each good laboratory and market, it is simple glass Büchner funnel (VLC column) with different filter frit (G1, G2, & G3). The people are using various types of column for natural products fractionation and isolation, but G1 is better as it can work at low vacuum pressure while in case of G2 &G3 (fine pore size) more suction pressure need to apply which results several losses of solvent. In contrast to other forced flow column chromatographic techniques, not pressure but vacuum is applied in VLC to increase flow rate and hence speed up the fractionation.
Acknowledgements
Authors are thankful to Pharmacopoeia Commission for Indian Medicine and Homeopathy (PCIM&H), Ghaziabad and Ministry of AYUSH, Govt. of India for providing necessary facilities to write this article.
References
- Bucar F, Wube A, Schmid M (2013) Natural product isolation-how to get from biological material to pure compounds. Nat Prod Rep 30(4): 525-545.
- Targett NM, Kilcoyne JP, Green B (1979) Vacuum liquid chromatography: an alternative to common chromatographic methods. J Org Chem 44(26): 4962-4964.
- Hostettmann K, Mardton A, Hostettmann M (1998) Preparative Chromatography Techniques. springer-verlag berlin heidelberg.
- Coll JC, Bowden BF (1986) The Application of Vacuum Liquid Chromatography to the Separation of Terpene Mixtures. J Nat Prod 49(5): 934-936.
- Maurya A (2014) Chemical investigation and development of validated analytical methods for some selected medicinal plants. RML Avadh University, PhD Thesis, Faizabad, India.
- Zubairi SI, Sarmidi MR (2004) Purification and identification of rotenone from Derris elliptica by using the vacuum liquid chromatography-thin layer chromatography (VLC-TLC) method. Proceeding of ‘Simposium Kimia Analisis Kebangsaan’, Swiss Garden Resort, Pahang.
- Yinusa I, Ilogbulem N, Ayo RG, Yakubu R (2015) Vacuum Liquid Chromatographic Isolation and Characterization of Beta-Sitosterol from the Bark of Sarcocephalus Latifolius (Smith Bruce). Journal of Chemistry and Chemical Sciences 5(9): 480-488.






























