Nonbranched-Chain Formation of 1,2-Alkanediols in Systems Alcohol-Formaldehyde
Michael M Silaev*
Chemistry Faculty, Lomonosov Moscow State University, Vorobievy Gory, Russia
Submission: May 02, 2018; Published: June 07, 2018
*Corresponding author: Michael M Silaev, Chemistry Faculty, Lomonosov Moscow State University, Vorobievy Gory, Moscow 119991, Russia, Email: mmsilaev@rc.chem.msu.ru
How to cite this article: M.M. Silaev. Nonbranched-Chain Formation of 1,2-Alkanediols in Systems Alcohol-Formaldehyde. Organic & Medicinal Chem IJ. 2018; 7(2): 555709. DOI: 10.19080/OMCIJ.2018.07.555709
Abstract
A mechanism of the initiated no branched-chain process of forming 1,2-alkanediols and carbonyl compounds in alcohol-formaldehyde sys-tems is suggested. The quasi-steady-state treatment is used to obtain kinetic equations that can describe the no monotonic (with a maximum) depend-ences of the formation rates of the products on the concentration of freeunsolvatedformaldehyde. The experimental concentration of the free unsolvated form of formaldehyde is given at the different temperatures, solvent permittivity, and total concentrations of formaldehyde in water and alcohols. An empirical equation for calculating the free formaldehyde concentration in alcohol-formaldehyde (including water/ethanediol-formaldehyde) systems at various temperatures and total formaldehyde concentrations and an equation for evaluating solvent concentrations in these systems were derived.
Keywords: No Branched-Chain Process; Free Formaldehyde; 1-Hydroxyalkyl Radical; Formyl Radical; Competing Reaction; Equation
Introduction
Free radicals add to the carbon atom at the double bond of the carbonyl group of dissolved free (unsolvated, monomer) formaldehyde. The concentration of free formaldehyde in the solution at room temperature is a fraction of a percent of the total formaldehyde concentration, which includes formaldehyde chemically bound to the solvent [1]. The concentration of free formaldehyde exponentially increases with in-creasing temperature [2]. The energy released as a result of this addition, when the C=O bond is converted into an ordinary bond, is 30 to 60 kJ mol-1 (according to the data on the addition of С1-С4 alkyl radicals in the gas phase under standard conditions [3-6]. The resulting free 1:1 adduct radicals can both abstract hydrogen atoms from the nearest neighbor molecules of the solvent or unsolvated formaldehyde and, due to its structure, decompose by a monomolecular mechanism including isomerization [7,8]. The analysis of stable liquid-phase products was carried out by the gas chromatographic method. The qua-si-steady-state treatment is used to obtain kinetic equations.
Addition of 1- Hydroxyalklyl Free Radicals With Two or More Carbon Atoms
Free 1-hydroxyalkyl radicals (which result from the abstraction of a hydrogen atom from the carbon atom bonded to the hydroxyl group in molecules of saturated aliphatic alcohols but methanol under the action of chemical initiators [9,10], light [11,12], or ionizing radiation [13,14] add at the double bond of free formaldehyde dissolved in the alcohol, forming 1,2-alkanediols [7-10,12-18], carbonyl compounds, and methanol [14,15] via the chaining mechanism. (The yields of the latter two products in the temperature range of 303 to 448 K are one order of magnitude lower). In these processes, the determining role in the reactivity of the alcohols can be played by the desolvation of formaldehyde in alcohol-formaldehyde solutions, which depends both on the temperature and on the polarity of the solvent [2,14]. For the γ-radiolysis of 1(or 2)-propanol-formaldehyde system at a constant temperature, the dependences of the radiation-chemical yields of 1,2-alkanediols and carbonyl compounds as a function of the formaldehyde concentration show maxima and are symbatic [13,15]. For a constant total formaldehyde concentration of 1 mol dm-3, the dependence of the 1,2-alkanediol yields as a function of temperature for 303-473 K shows a maximum, whereas the yields of car-bonyl compounds and methanol increase monotonically [14] (along with the concentration of free formaldehyde [2]. In addition to the above products, the nonchain mechanism in the γ -radiolysis of the solutions of formaldehyde in ethanol and 1- and 2-propanol gives ethanediol, carbon monoxide, and hydrogen in low radiation-chemical yields (which, however, exceed the yields of the same products in the γ -radiolysis of individual alcohols) [7,14,15]. The available experimental data can be described in terms of the following scheme of reactions (Scheme).
Chain Initiation

Inhibition

Chain Termination


In these reactions, I is an initiator, e.g., a peroxide [9,10]; 0R, some reactive radical (initiator radical); R, an alkyl; ROH, a saturated aliphatic alcohol, either primary or secondary, beginning from ethanol; CH2O, the unsaturated molecule - free formaldehyde; СН2ОН, the 1-hydroxymetyl fragment radical; R(-H)OH, the reactive 1-hydroxyalkyl addend radical, beginning from 1-hydroxyethyl; R(-H)(ОH)СН2О, the reactive hydroxyalkoxyl 1:1 adductradical; СНО, the low-reactive formyl radical (inhibitor radical); R0H, the molecular product; R(-H)(OH)СН2ОН, 1,2-alkanediol; R(-2H) HO, an aldehyde in the case of a primary alcohol and an R’R”CO ketone in the case of a secondary alcohol; R(-H)(ОH)R( H)ОH, a vicinal alkanediol;R(-H)(OH)CHO, a hydroxyaldehyde. The chain evolution stage of Scheme includes consecutive reaction pairs 2-3, 2-3a, and 3a-3b; parallel (competing) reaction pairs 3-3a, 3-3b, 3-4, and 3a-4; and consecutive-parallel reactions 2 and 4.
In addition, it seems unlikely that free adduct radicals will add to formaldehyde at higher temperatures the reaction of adding is unlikely because this would result in an ether bond. The addition of hydroxymethyl radicals to formaldehyde, which is in competition with reaction 3b, is not included as well, because there is no chain formation of ethanediol at 303-448 K [14]. At the same time, small amounts of ethanediol can form via the dimerization of a small fraction of hydroxymethyl radicals, but this cannot have any appreciable effect on the overall process kinetics. The addition of free formyl radicals to formaldehyde cannot proceed at a significant rate, as is indicated by the fact that there is no chain formation of glycol aldehyde in the systems examined [14]. The mechanism of the decomposition of the free adduct radical via reaction 3a, which includes the formation of an intramolecular НО bond and isomerization, can be represented as follows [7,8,15]:
The probability of the occurrence of reaction 3a should increase with increasing temperature. This is indicated by experimental data presented above [7,8,15]. The decomposition of the hydroxyalkoxyl radical. R(-H)(ОH)СН2О (reaction 3a) is likely endothermic. The endothermic nature of reaction 3a is indirectly indicated by the fact that the decomposition of simple C2-C4 alkoxyl radicals RО in the gas phase is accompanied by heat absorption: (ΔH0 298 = 30-90 kJ mol-1 [4-6]). Reaction 3b, subsequent to reaction 3a, is exothermic, and its heat for C2-C3 alcohols in the gas phase is ΔH0 298 = -40 to -60 kJ mol-1 [4-6]. As follows from the above scheme of the process, reactions 3a and 3b, in which the formation and consumption of the highly reactive free radical hydroxymethyl take place (at equal rates under steady-state conditions), can be represented as a single bimolecular reaction 3a,b occurring in a “cage” of solvent molecules. The free formyl radical resulting from reaction 4, which is in competition with reactions 3 and 3a, is comparatively low-reactive because its spin density can be partially delocalized from the carbon atom via the double bond toward the oxygen atom, which possesses a higher electron affinity [3]. For example, in contrast to the methyl and alkoxyl π-radicals, the formyl σ-radical can be stabilized in glassy alcohols at 77K [19].
In the gas phase, the dissociation energy of the C-H bond in formyl radicals is half that for acetyl radicals and is about 5 times lower than the dissociation energy of the Сα-Н bond in saturated C1-C3 alcohols [3]. As distinct from reactions 3 and 3a,b, reaction 4 leads to an inefficient consumption of hydroxyalkoxyl adduct radicals, without regenerating the initial 1-hydroxyalkyl addend radicals. Reaction 4 together with reaction 6 (mutual annihilation of free formyl and chain-carrier 1-hydroxyalkyl radicals) causes the inhibition of the no branched-chain process. For the disproportionation of the free radicals, the heats of reactions 5-7 for C1-C3 alcohols in the gas phase vary in the range of Δ H0 298 = -135 to -385 kJ mol-1 [3-6]. The rates of the chain formation of 1,2-alkanediols in reaction 3 (and their nonchain formation in reaction 4), carbonyl compounds in reaction 3a, and methanol in reaction 3b are given by the following equations1:

where V1 is the initiation rate, l is the molar concentration of the saturated alcohol at the given total concentration c0 of formaldehyde2 dissolved in it, x is the molar concentration of free formaldehyde (l >> x), k2 is the rate constant of reaction 2 (addition of 1-hydroxyalkyl free radical to free formaldehyde), and α = k3/k4 and β = k3а/k4 (mol dm-3) are the ratios of the rate constants of the competing (parallel) reactions. Estimates of 2k5 were reported by Silaev et al. [20,21]. From the extremum condition for the reaction 3a rate function, ∂ V3a / ∂х = 0, we derived the following analytical. Equations (1) and (2) taken on the condition that k2x2 >>(αl + x) 5 1 2k V (the descending branch of the curve having a maximum) can be transformed to equations that can be
used for a preliminary estimation of parameters α and β:

Where φ = 2 for the maximum (when  ) and
φ = 1 for the descending portion of the curve. The rate equations
were derived by quasi-steady-state treatment, which is most
suitable for describing the processes including at least eight to ten
reactions with four to six different free radicals and at most three
to seven experimental points in their functional curves, using the
condition for the first steps of the process that makes it possible to
reduce the exponent of term 2k5[R(−H)OH]2 to 1 in equation d[R]/
dt = 0 [7,15]: k6 =
) and
φ = 1 for the descending portion of the curve. The rate equations
were derived by quasi-steady-state treatment, which is most
suitable for describing the processes including at least eight to ten
reactions with four to six different free radicals and at most three
to seven experimental points in their functional curves, using the
condition for the first steps of the process that makes it possible to
reduce the exponent of term 2k5[R(−H)OH]2 to 1 in equation d[R]/
dt = 0 [7,15]: k6 =  and V1 = V5 + 2V6 + V7 =
and V1 = V5 + 2V6 + V7 =  . The relations between the reaction rates and
radiation-chemical yields for radiation- chemical processes are
reported by Silaev [23].
. The relations between the reaction rates and
radiation-chemical yields for radiation- chemical processes are
reported by Silaev [23].
The alcohol concentration in alcohol-formaldehyde solutions at any temperature can be estimated by the method suggested in [20,24]. The data necessary for estimating the concentration of free formaldehyde using the total formaldehyde concentration in the solution are reported by Silaev et al. [2, 20]. The overall process rate is a complicated function of the formation and disappearance rates of the R(-H)OH and СНО free radicals:

The ratios of the rates of the competing reactions are V3/V4 = αl/x and V3a/V4 = β/x, and the chain length is v = (V3 +V3a)/V1. The ratio of the rates of formation of 1,2-alkanediol and the carbonyl compound is a simple linear function of x:

The equations for the rates of chain-termination reactions 5-7 are given by the following equations:

where

Neutral formaldehyde solutions in alcohols at room temperature primarily consist of a mixture of formaldehyde polymer solvates reversibly bound to alcohols; these polymer solvates differ in molecular mass and have the general formula RO(CH2O)nH, where n = 1-4 [1]. The concentration of formaldehyde that occurs in solution as a free, unsolvated active species chemically unbound to the solvent (this species is capable of scavenging free radicals) at room temperature is lower than a percent of the total formaldehyde concentration [1]. The concentration x of the free formaldehyde species in solutions was determined by high-temperature UV spectrophotometry in the range 335-438 K at the total formaldehyde concentration c0 (free and bound species including the concentration of polymer solvates) of 1.0-8.4 mol dm-3 in water, ethanediol, methanol, ethanol, 1-propanol, 2-propanol, and 2-methyl-2-propanol [2] (see Table of the Appendix 1). This concentration increases with temperature according to an exponential law, and it can be as high as a few percent of the total concentration in solution under the test conditions, up to 19.3% in the case of 2-methyl-2-propanol at a total concentration of 1.0 mol dm-3 and a temperature of 398 K.
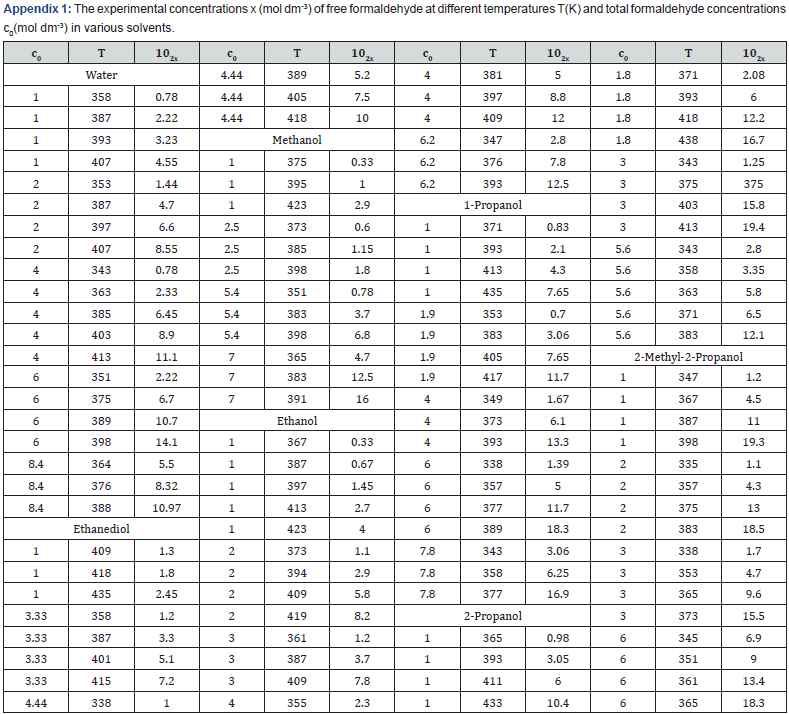
The following empirical equation relating the concentration x (mol dm-3) of free formaldehyde to temperature T (K) and the total concentration c0 in the solution (measured at room temperature), was developed by the treatment of 101 data points [2,20]:

where the coefficients a and b were calculated as the parameters of a straight-line equation by the least-squares technique from the dependence of lg x on 1/T at c0 = 1.0 mol dm-3 for various solvents, and the coefficient h was obtained as the average value of the slopes of lg x as linear functions of lg c0 at various series of fixed temperatures. The Table 1 summarizes these coefficients for each solvent. As regards the experimental data, the error in the calculations of the concentration x of free formaldehyde made by Equation (8) in the specified temperature range was no higher than 25%. On the assumption that the dependence of the density of a given solution on the concentration of formaldehyde is similar to the analogous linear dependence found for aqueous formaldehyde solutions (0-14 mol dm-3; 291 K) [25], the concentrations lT (mol dm-3) of alcohols in alcohol-formaldehyde solutions at a certain temperature can be estimated by the equation,

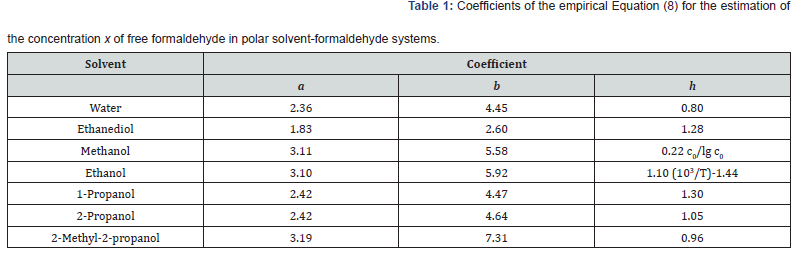
where c0 is the total formaldehyde concentration (mol dm-3); M is the molecular mass (g mol-1) of the solvent; d and dT are the solvent densities (g cm-3) at room and given temperatures, respectively; the coefficients 8.4 × 10-3 and 21.6 have the units of 103g mol-1 and g mol-1, respectively. Earlier [2], it was found that the concentration x of the free formaldehyde species decreased with the solvent permittivity D298 at a constant temperature. Water is an exception. Although water is more polar than alcohols, the concentration x of free formaldehyde in an aqueous solution is anomalously high and reaches the level of its concentration in 2-propanol, all other factors being the same (Figure 1) [2,20]. This can be due to the specific instability of hydrated formaldehyde species and the ease of their conversion into free formaldehyde with increasing temperature.


As an example, the Figure 2 illustrates the use of Equations (1) & (2) for describing the experimental dependences of the formation rates of 1,2-butanediol (curve 1) in reactions 3 and 4 and propanal (curve 2) in reaction 3a on the concentration x of free formaldehyde in the 1-propanol-formaldehyde reacting system at the total formaldehyde concentration c0 of 2.0 to 9.5 mol dm-3 and temperature of 413K [7,15,26]. The concentration dependence of the propanal formation rate was described using the estimates of kinetic parameters obtained for the same dependence of the 1,2-butanediol formation rate. We considered these data more reliable for the reason that the carbonyl compounds forming in the alcohol-formaldehyde systems can react with the alcohol and this reaction depends considerably on the temperature and acidity of the medium [1]. The mathematical modeling of the process was carried out using a 137Cs γ-radiation dose rate of P = 0.8 Gy s-1 [13,26], a total initiation yield of G(CH3СН2ĊНОН) = 9.0 particles per 100 eV [7,15] (V1 = 4.07 × 10-7 mol dm-3 s-1), and 2k5 = 4.7 ×109 dm3 mol-1 s-1. The following values of the parameters were obtained: α = 0.36 ± 0.07, β = 0.25 ± 0.05 mol dm-3, and k2 = (6.0 ± 1.4) ×103 dm3 mol-1 s-1.
Note that, as compared to the yields of 1,2-propanediol in the γ-radiolysis of the ethanol-formaldehyde system, the yields of 2,3-butanediol in the γ-radiolysis of the ethanol-acetaldehyde system are one order of magnitude lower [26]. Using data from [7,15], it can be demonstrated that, at 433 K, the double bond of 2-propen-1-ol accepts the 1-hydroxyethyl radical 3.4 times more efficiently than the double bond of formaldehyde [27].
Addition of Hydroxymethyl Free Radicals
The addition of hydroxymethyl radicals to the carbon atom at the double bond of free formaldehyde molecules in methanol, initiated by the free-radical mechanism, results in the chain formation of ethanediol [16]. In this case, reaction 3a in Scheme is the reverse of reaction 2, the 1-hydroxyalkyl radical R(-H)OH is the hydroxymethyl radical СН2ОН, so reaction 3b is eliminated (k3b = 0), and reaction 5 yields an additional amount of ethanediol via the dimerization of chain-carrier hydroxymethyl radicals (their disproportionation can practically be ignored [28]. The scheme of these reactions is presented in [17]. The rate equation for ethanediol formation by the chain mechanism in reaction 3 and by the nonchain mechanism in reactions 4 and 5 in the methanolformaldehyde system has a complicated form 3 as compared to Equation (1) for the formation rate of the other 1,2-alkanediols [8]:

where 
Equation (10) when k2x2 << (αl + β + x) 51 2kV (ascending branch of the curve having a maximum) and αl >> β (practically without reaction 3a) is transformed to a simple directly-proportional dependence on the concentration x of free formaldehyde, which can be used to pre-estimate the parameter k2:

where φ = 1 for the ascending portion of the curve and φ =
2 for the maximum, when k2x2 ≅ (αl + β + x)  If the rate of ethanediol formation by the dimerization mechanism in reaction
5 is ignored for the reason that it is small as compared to the
total rate of ethanediol formation in reactions 3 and 4, Equation
(10) will be identical to Equation (1). After the numerator and
denominator on the right-hand side of Equation (1) are divided by
k-2 ≡ k3a, one can replace k2 in this equation with k2 = k2/k-2, which
is the equilibrium constant for the reverse of reaction 2. Ignoring
the reverse step of reaction 2 (k3a = 0, β = 0) can further simplify
Equation (1). In this case, the rate constant k2 is effective.
If the rate of ethanediol formation by the dimerization mechanism in reaction
5 is ignored for the reason that it is small as compared to the
total rate of ethanediol formation in reactions 3 and 4, Equation
(10) will be identical to Equation (1). After the numerator and
denominator on the right-hand side of Equation (1) are divided by
k-2 ≡ k3a, one can replace k2 in this equation with k2 = k2/k-2, which
is the equilibrium constant for the reverse of reaction 2. Ignoring
the reverse step of reaction 2 (k3a = 0, β = 0) can further simplify
Equation (1). In this case, the rate constant k2 is effective.
References
- S K Ogorodnikov (1984) Formal’degid (Formaldehyde), Khimiya, Leningrad.
- MM Silaev, AV Rudnev, EP Kalyazin (1989) Formaldehyde III. Concentration of Free Formaldehyde as a Function of Temperature, Polarity of Solvents, and Total Concentration of Formaldehyde in Solution. Zhurnal Fizicheskoi Khimii 53: 1647-1651.
- LV Gurvich, GV Karachevtsev, VN Kondrat’ev, Yu A Lebedev, VA Medvedev, et al. (1974) Energii razryva khimicheskikh svyazei. Potentsialy ionizatsii i srodstvo k elektronu. (“Bond Dissociation Energies, Ionization Potentials, and Electron Affinity”). VN Kondrat’ev, Nauka, Moscow.
- SW Benson (1976) Thermochemical Kinetics: Methods for the Estimation of Thermochemical Data and Rate Parameters. (2nd Edn.); Wiley, New York, USA.
- JB Pedley, RD Naylor, SP Kirby (1986) Thermochemical Data of Organic Compounds. (2nd Edn.); Chapman Hall, London, UK.
- Yu D Orlov, Yu A Lebedev, I Sh Saifullin (2001) Termokhimiya organicheskikh svobodnykh radikalov (“Thermochemistry of Organic Free Radicals”). AM Kutepov, Nauka, Moscow, USA.
- MM Silaev, LT Bugaenko (1994) Kinetics of the Addition of α-Hydroxyalkyl Radicals to 2-Propen-1-ol and Formaldehyde. Kinetics and Katalysis 35: 509-513.
- MM Silaev (2007) Simulation of Nonbranched Chain Processes for Producing 1,2-Alkanediols in Alcohol-Formaldehyde Systems. Thoretical Foundations Chemical Engineering 41: 357-361.
- Oyama M (1965) A Free-Radical Reaction of Primary and Secondary Alcohols with Formaldehyde. The Journal of Organic Chemistry 30: 2429-2432.
- GI Nikishin, D Lefor, ED Vorob’ev (1966) Free Radical Reaction of Primary Alcohols with Formaldehyde. Izvestiya Akademii Nauk SSSR, Ser Khimiya 7: 1271-1272.
- WH Urry, FW Stacey, ES Huyser, OO Juveland (1954) The Peroxide- and Light-Induced Additions of Alcohols to Olefins. Journal of the American Chemical Society 76: 450-455.
- MB Dzhurinskaya, AV Rudnev, EP Kalyazin (1984) High Temperature UV Photolysis of Formaldehyde in Liquid Methanol. Vestnik Moskovskogo Universiteta, Ser 2: Khimiya 25: 173-176.
- EP Kalyazin, EP Petryaev, OI Shadyro (1977) Reaction between Oxyalkyl Radicals and Aldehydes. Zhurnal Organicheskoi Khimii 13: 293-295.
- AI Novoselov, Silaev MM, LT Bugaenko (2004) Effect of Temperature on the Yields of Final Products in the γ-Radiolysis of Formaldehyde Solutions in C1-C3 Alkanols. High Energy Chemistry 38: 236-238.
- MM Silaev, LT Bugaenko (1992) Mathematical Simulation of the Kinetics of Radiation Induced Hydroxyalkylation of Aliphatic Saturated Alcohols. Radiation Physics and Chemistry 40: 1-10.
- AI Novoselov, MM Silaev, LT Bugaenko (2008) Dependence of Ethanediol Yield on Formaldehyde Concentration in γ-Radiolysis of Methanol-Formaldehyde System at 373-473K. High Energy Chemistry 42: 69-70.
- AI Novoselov, MM Silaev, LT Bugaenko (2010) γ-Induced Single-Step Synthesis of Ethylene Glycol from Methanol-Formaldehyde Solution. Theoretical Foundation of Chemical Engineering 44: 432-435.
- AI Novoselov, MM Silaev, LT Bugaenko (2007) Dependence of 1,2-Propanediol Yield on Formaldehyde Concentration in γ-Radiolysis of Ethanol-Formaldehyde System at 373-473 K. High Energy Chemistry 41: 53.
- S Ya Pshezhetskii, AG Kotov, VK Milinchuk, VA Roginskii, VI Tupikov (1972) EPR svobodnykh radikalov v radiatsionnoi khimii. ESR of Free Radicals in Radiation Chemistry, Khimiya, Moscow, USA.
- MM Silaev (2002) Applied Aspects of the γ-Radiolysis of C1-C4 Alcohols and Binary Mixtures on Their Basis. High Energy Chemistry 36: 70-74.
- MM Silaev, LT Bugaenko, EP Kalyazin (1986) On the Possibility of Adequately Estimating of the Rate Constants for the Reaction of Hydroxyalkyl Radicals with Each Other Using the Self-Diffusion Coefficients or Viscosities of the Corresponding Alcohols. Vestnik Moskovskogo. Univiversiteta Ser 2: Khimiya 27: 386-389.
- L Bateman (1954) Olefin Oxidation. Quarterly Reviews 8: 147-167.
- MM Silaev (2007) Simulation of the Nonbranched-Chain Addition of Saturated Free Radicals to Alkenes and Their Derivatives Yielding 1:1 Adducts. Theoretical Foundations of Chemical Engineering 41: 273- 278.
- MM Silaev (1993) Estimating the Solvent Concentration in Formaldehyde Solutions at Various Temperatures. Zhurnal Fizicheskoy Khimii 67: 1944.
- JF Walker (1957) Formaldehyde, Reinhold, New York, 1953, English Translation under the title Formal’degid. Goskhimizdat Moscow pp: 106.
- OI Shadyro (1975) Radiation-chemical Conversions of Aldehydes in Various Systems. Chemistry, Belarusian State University, Minsk.
- MM Silaev (1993) Relative Reactivity of α-Hydroxyethyl Radicals for 2-Propene-1-ol and Formaldehyde Double-Bond Addition. Vestnik Moskovskogo Universiteta Ser. 2: Khimiya 34: 311.
- H Seki, R Nagai, M Imamura (1968) γ-Radiolysis of a Binary Mixture of Methanol and Water: The Formation of Formaldehyde in the Radiolysis of Liquid Methanol. Bulletin of the Chemical Society of Japan 41: 2877- 2881.






























