A Brief Review on Transdermal Patches
Nidhi Sharma*
HIMT College of Pharmacy, Greater Noida, India
Submission: May 12, 2018; Published: June 05, 2018
*Corresponding author: Nidhi Sharma, HIMT College of Pharmacy, Greater Noida, India, Email: nidhishar27@gmail.com
How to cite this article: Nidhi S. A Brief Review on Transdermal Patches. Organic & Medicinal Chem IJ. 2018; 7(2): 555707. DOI: 10.19080/OMCIJ.2018.07.555707
Abstract
Transdermal drug delivery system was presented to overcome the difficulties of drug delivery especially oral route. A transdermal patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream. It promotes healing to an injured area of the body. An advantage of a transdermal drug delivery route over other types of delivery system such as oral, topical, i.v., i.m., etc. is that the patch provides a controlled release of the medication into the patient, usually through either a porous membrane covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier, as a result, only medications whose molecules are small can easily penetrate the skin, so it can be delivered by this method. This review article describes the overall introduction of transdermal patches including type of transdermal patches, method of preparation of transdermal patches and factor affecting etc.
Keywords:Transdermal drug delivery system; Hydrin rubber; Silicon rubber; Polyvinylalcohol; Transdermal patch; Polyvinylchloride; Di-N-butylphthalate; Triethylcitrate
Abbrevations:PE: Polyethelene; EVAC: Ethylene Vinyl Acetate Copolymer; PSA: Pressure Sensitive Adhesive
Introduction
Oral route is the most popular route of drug delivery system but it has some disadvantages including first pass metabolism, drug degradation etc in gastrointestinal tract due to enzymes, pH etc. To overcome these problems, a novel drug delivery system was developed by Chien in 1992, Banker in 1990, Guy in 1996. It was Transdermal patches or Transdermal delivery system. In this system medicated adhesive patches are prepared which deliver therapeutically effective amount of drug across the skin when it placed on skin. They are available in different sizes & having more than one ingredient. Once they apply on unbroken skin they deliver active ingredients into systemic circulation passing via skin barriers. A transdermal patch containing high dose of drug inside which is retained on the skin for prolonged period of time, which get enters into blood flow via diffusion process.
Drug can penetrate through skin via three pathways-
a) Through hair follicals.
b) Through sebaceous glands
c) Through sweat duct.
Transdermal drug delivery systems are used in various skin disorders, also in the management of angina pectoris, pains, smoking cessation & neurological disorders such as Parkinson’s disease [1,2].
Types of Transdermal Drug Delivery System
Single-layer Drug-in-Adhesive System: In this type of patch the adhesive layer of this system contains the drug. The adhesive layer not only serves to adhere the various layers together, along with the entire system to the skin, but it is also responsible for the releasing the drug. The adhesive layer is surrounded by a temporary liner and a backing (Figure 1).
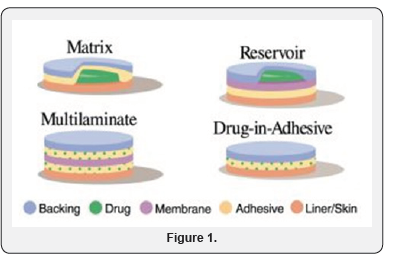
Reservoir System: In this System the drug reservoir is kept in between backing layer and a rate controlling membrane. And drug releases through microporous rate controlled membrane. Drug can be in the form of a solution, suspension,or gel or dispersed in a solid polymer matrix in the reservoir compartment.
Matrix System: This system is of Two type
a) Drug-in-Adhesive System: For the formation of drug reservoir, the drug dispersed in an adhesive polymer and then spreading the medicated polymer adhesive by solvent casting or by melting the adhesive (in the case of hot-melt adhesives) on to an impervious backing layer.
b) Matrix-Dispersion System: In this system the drug is dispersed homogeneously in a hydrophilic or lipophilic polymer matrix. And this containing polymer along with drug is fixed onto an occlusive base plate in a compartment fabricated from a drug- impermeable backing layer. In this system the adhesive is spread along the circumference instead of applying on the face of drug reservoir to form a strip of adhesive rim [3].
Micro-Reservoir System: This system is a combination of reservoir and matrix- dispersion systems. In which drug is suspended in an aqueous solution of water-soluble polymer and then dispersing the solution homogeneously in a lipophilic polymer to form thousands of unleachable, microscopic spheres of drug reservoirs [4].
Components of Transdermal Drug Delivery System
a) Polymer matrix/ Drug reservoir
b) Drug
c) Permeation enhancers.
d) Pressure sensitive adhesive (PSA).
e) Backing laminate
f) Release liner.
g) Other excipients like plasticizers and solvents [5].
Polymer Matrix/ Drug Reservoir: It is prepared by dispersing the drug in liquid or solid state synthetic polymer base. It should have biocompatibility and chemical compatibility with the drug and other components of the system such as penetration enhancers. Additionally they should provide consistent and effective delivery of a drug throughout the product’s intended shelf life and should be of safe status. Polymers used in Transdermal drug delivery systems are classified as
a) Natural Polymers: e.g. cellulose derivatives, zein, gelatin, shellac, waxes, gums, natural rubber and chitosan etc.
b) Synthetic Elastomers: e.g. polybutadiene, hydrin rubber, silicon rubber, polyisobutylene, acrylonitrile, neoprene, butyl rubber etc.
c) Synthetic Polymers: e.g. polyvinylalcohol, polyvinylchloride, polyethylene, polypropylene, polyacrylate, polyamide, polyurea, polyvinylpyrrolidone, polymethylmethacrylate etc [6,7].
Drugs: Some of ideal properties of drug & some factors to be consider during preparation of Transdermal patches are as follows:
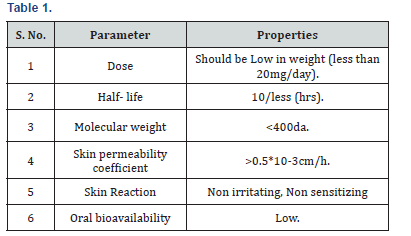
a. Ideal Properties of Drugs: (Table 1)
b. Factors Affecting: (Table 2)

Permeation Enhancers: The chemical compounds that enhance the permeability of stratum corneum so as to attain therapeutic levels of the drug candidate. They improve the permeability by interacting with Stratum corneum.
a) Ideal Properties of Permeation Enhancers
i. They should be non-irritating, non-toxic & non- allergic
ii. They should not bind to receptor site i.e. not showing any pharmacological activity.
iii. They should be cosmetically acceptable with an appropriate skin feel. [8]
Pressure Sensitive Adhesive (PSA): It helps to increase the adherence of transdermal patch to the skin surface. It can easily remove from the smooth surface without leaving a residue on it.
a) Polyacrylates
b) Polyisobutylene
c) silicon based adhesives
Backing Laminate: It is a supportive material which is impermeable to drugs and also to permeation enhancers. They should chemically compatible with the drug, enhancer, adhesive and other excipients.
Ex: Vinyl, Polyethylene and Polyester films [9].
Release Liner: This is the primary packaging material that can protect the patch during application. It is made up of base layer which may be
a) Non-occlusive (e.g. paper fabric)
OR
b) Occlusive (e.g. polyethylene, polyvinylchloride)
It is made up of silicon or Teflon. Release liner should be chemically inert & it should be permeable to drug, penetration enhancers & water.
Other Excipients Like Plasticizers and Solvents
a) Solvents: Chloroform, methanol, acetone, isopropanol and dichloromethane.
b) Plasticizers: Dibutylpthalate, triethylcitrate, polyethylene glycol and propylene glycol [10].
Methods of Preparation of TDDS
a) Asymmetric TPX membrane method.
b) Circular Teflon mould method.
c) Mercury substrate method.
d) By using “IPM membranes” method.
e) By using “EVAC membranes” method.
f) Preparation of TDDS by using Proliposomes.
g) By using free film method.
Asymmetric TPX Membrane Method: This method was discovered by Berner and John in 1994. By this method prototype patch can be prepared by using heat sealable polyester film (type 1009, 3m) with a concave of 1cm diameter as the backing membrane. Drug dispersed on concave membrane, covered by a TPX [poly (4-methyl-1- pentene)] asymmetric membrane, and sealed by an adhesive.
a) Preparation: These are prepared by using the dry or wet inversion process. In this TPX is dissolved in a mixture of solvent (cyclohexane) and non- solvent additives at 60°C to form a polymer solution. The polymer solution is kept at 40°C for 24 hrs and cast on a glass plate. Then casting film is evaporated at 50°C for 30 sec, then the glass plate is to be immersed immediately in coagulation bath (temperature mantained at 25°C). After 10 minutes of immersion, the membrane can be removed, air dry in a circulation oven at 50°C for 12 hrs.
Circular Teflon Mould Method: It was discovered by Baker and Heller in 1989. Polymeric solution in various proportions is used as an organic solvent. Then that solution is divided in two parts. In one parts calculated amount of drug is dissolved & in another part enhancers in different concentration are dissolved, and then two parts mixed together. Then plasticizer (e.g., Di-Nbutylphthalate) is added into the drug polymer solution. The total contents are to be stirred for 12 hrs and then poured into a circular Teflon mould. The moulds are to be placed on a levelled surface and covered with inverted funnel to control solvent vaporization in a laminar flow hood model with an air speed of 0.5 m/s. The solvent is allowed to evaporate for 24 h. After which a dried film formed & that is to be stored for another 24 h at 25±0.5°C in a desiccators containing silica gel before evaluation to eliminate aging effects.
Mercury Substrate Method: In this method drug & plasticizer get dissolved in polymeric solution. It stirred for 10- 15 min to produce homogenous dispersion then it is poured into levelled mercury surface, covered with inverted funnel to control solvent evaporation.
By Using “IPM Membranes” Method: In the mixture of water & polymer (propylene glycol containing Carbomer 940 polymer) drug get dispersed and stirred for 12 hrs in magnetic stirrer. The dispersion is to be neutralized and made viscous by the addition of triethanolamine. If the drug solubility in aqueous solution is very poor then solution gel is obtained by using Buffer pH 7.4. The formed gel will be incorporated in the IPM membrane.
By Using “EVAC Membranes” Method: For the preparation of TDS, 1% carbopol reservoir gel, polyethelene (PE), ethylene vinyl acetate copolymer (EVAC) membrane is needed as rate control membrane. If the drug is insoluble in water then use propylene glycol for gel preparation. Drug is dissolved in propylene glycol, carbopol resin will be added to the above solution and neutralized by using 5% w/w sodium hydroxide solution. The drug (in gel form) is placed on a sheet of backing layer covering the specified area. A rate controlling membrane will be placed over the gel and the edges will be sealed by heat to obtain a leak proof device.
Preparation of TDDS by Using Proliposomes: By carrier method using film deposition technique proliposomes are prepared. Drug and lecithin ratio should be 0.1:2.0 taken as an optimized one from previous references. For the preparation of proliosome in 100ml round bottom flask take 5mg of mannitol powder, then it is kept at 60-70°c temperature and the flask is rotated at 80-90 rpm and dried the mannitol at vacuum for 30 minutes. After drying, the temperature of the water bath is adjusted to 20- 30°C. Drug and lecithin are dissolved in a suitable organic solvent mixture, a 0.5ml aliquot of the organic solution is introduced into the round bottomed flask at 37°C, after complete drying second aliquots (0.5ml) of the solution is to be added. After the last loading, the flask containing proliposomes are connected in a lyophilizer and subsequently drug loaded mannitol powders (proliposomes) are placed in a desiccator overnight and then sieved through 100 mesh. The collected powder is transferred into a glass bottle and stored at the freeze temperature until characterization.
By using Free Film Method: In this process firstly cellulose acetate free film is prepared by casting it on mercury surface. And 2% w/w polymer solution is prepared by using chloroform. Plasticizers are to be added at a concentration of 40% w/w of polymer weight. Then 5 ml of polymer solution is poured in a glass ring which is placed over the mercury surface in a glass petridish. The rate of evaporation of the solvent can be controlled by placing an inverted funnel over the petridish. The film formation is noted by observing the mercury surface after complete evaporation of the solvent. The dry film will be separated out and stored between the sheets of wax paper in a desiccator until use. By this process we can prepare free films of different thickness can be prepared by changing the volume of the polymer solution [11,12].
Factors Affecting Transdermal Patches
There are various factors which affects the action of transdermal patches. These are given below:
a. Physicochemical Properties
i. Partition coefficient
ii. Molecular size
iii. Solubility/melting point
iv. Ionization
b. Physiological & Pathological Conditions of Skin
i. Reservoir effect of horny layer
ii. Lipid film
iii. Skin hydration
iv. Skin temperature
v. Regional variation
vi. Pathological injuries to the skin
vii. Cutaneous self-metabolism
viii. Skin barrier properties in the neonate and young infant
ix. Skin barrier properties in aged skin
x. Race
xi. Body site
xii. Penetration enhancers used [13]
Advantages
a) First pass metabolisms of drug get avoided.
b) Gastrointestinal incompatibilities get avoided.
c) Self-medication is possible.
d) Duration of action gets extended & predictable.
e) Unwanted side effects get minimized.
f) Drug plasma concentration gets maintained.
g) Number of doses get reduces which improve patient compliance.
h) Therapeutic value of many drugs get increased by avoiding problems associated with drug like-lower absorption, GI irritation, decomposition due to hepatic first pass metabolism [14,15].
Disadvantages
a. Chances of allergic reactions at the site of application like- itching, rashes, local edema etc.
b. Larger molecular size of drug (above 1000) creates difficulty in absorption.
c. Barrier function of skin varies from site to site on the same or different person.
d. Drug with hydrophilic character is less suitable as compare to drug with lipophilic character because of their low permeability [16].
Future of Transdermal Drug Delivery System
Future aspects in Drug delivery system include liposomes, Niosomes and micro emulsion. Aim of this development is to improve delivery of drug that has low inherent solubility in most of classical formulation excipients. A wide range of potential drugs for delivery like steroids, antifungal, antibacterial, interferon, methotrexate, local anesthetics are formulated. The market for transdermal patches has been estimated to increase in future and has recently experienced annual growth of at rate of 25%. This figure will increase in future as novel devices emerge and list of marketed transdermal drug increases. Transdermal delivery of analgesics is likely to continue to increase in popularity as there are further improvements in design. Research is being performed to increase safety and efficacy. To improve practical matters such as the experience for the wearer of the patch, and also to provide more precise drug delivery associated with increased duration of action. Other potential improvements include improved transdermal technology that utilizes mechanical energy to increase drug flux across the skin either by altering the skin barrier or increasing the energy of the drug molecules. After the successful design of patches using iontophoresis, various modes of ‘active’ transdermal technologies are being investigated for different drugs. These include electroporation (short electrical pulses of high voltage to create transient aqueous pores in the skin), sonophoresis (uses low frequency ultrasonic energy to disrupt the stratum corneum), and thermal energy (uses heat to make the skin more permeable and to increase the energy of drug molecules). Magnetic energy, magnetophoresis, has been investigated as a means to increase drug flux across the skin. The transdermal patch may be an underutilized tool for management of acute and chronic pain. With improved delivery and a wider range of analgesics, we expect the popularity and applicability of this modality to deliver drugs to increase. In current scenario, transdermal route of drug delivery system in comparison with oral treatment as the most successful innovative research area in new drug delivery system, with around 40% of the drug delivery candidate products under clinical trails related to transdermal or dermal system. The transdermal drug delivery systems (TDDS) have been designed as an alternative, safest and easy route for systemic drug delivery. The systemic drug administration though skin holds several advantages such as maintenance constant drug level in blood plasma, less number of side effects, and improvement of bio availability by circumvention of hepatic first pass metabolism and increase patient compliance with respect to drug regime used for treatment. In recent times, skin considered as a safest port for drug administration, to provide continuous drug release into systemic circulation [17].
References
- Arti Kesarwani, Ajit Kumar Yadav, Sunil Singh, Hemendra Gautam, Haribansh N Singh, et al. (2013) A review-Theoretical aspects of Transdermal Drug Delivery System. Bulletin of Pharmaceutical Research 3(2): 78-89.
- Sampath Sampath Kumar KP, Debjit Bhowmik, Chiranjib B, RM Chandira (2010) A review- Transdermal Drug Delivery System- A Novel Drug Delivery System and its market scope and opportunities. International Journal of Pharma and Bio Sciences 1(2).
- Saurabh Pandey, Ashutosh Badola, Ganesh Kumar Bhatt, Preeti Kothiyal (2013) An Overview on Transdermal Drug Delivery System. International Journal of Pharmaceutical and Chemical sciences 2(3).
- P K Gaur, S Mishra, S Purohit, K Dave (2009) Transdermal Drug Delivery System: AReview. Asian Journal of Pharmaceutical and Clinical Research 2(1): 14-20.
- Vandana Yadav, Sipia Altaf Bhai M, Mamatha Y, Prashant VV (2012) Transdermal Drug Delivery System: A Technical Writeup. Journal of Pharmaceutical & Scientific innovation 1(1).
- Nikhil Sharma, Bharat Parashar, Shalini Sharma, Uday Mahajan (2012) Blooming Pharma Industry with Transdermal Drug Delivery System. Indo Global Journal of Pharmaceutical Sciences 2(3): 262-278.
- Saurabh Pandey, Ashutosh Badola, Ganesh Kumar Bhatt, Preeti Kothiyal an Overview on Transdermal Drug Delivery System. International Journal of Pharmaceutical and Chemical sciences.
- Kamal Gandhi, Anu Dahiya, Monika, Taruna Karla, Khushboo Singh Transdermal drug delivery-A Review.
- K Ezhumalai, P Ilavarasan, R Murali Mugundhan, U Sathiyaraj, AN Rajalakshmi (2011) Transdermal Patches in Novel Drug Delivery System. International Journal of Pharmacy & Technology 3(2): 2402- 2419.
- Hiren J Patel, Darshan G Trivedi, Anand K Bhandari, Dushyant A Shah (2011) Penetration enhancers for Transdermal Drug Delivery System: A Review. IJPI’s Journal of Pharmaceutics and Cosmetology 1(2).
- J Ashok Kumar, Nikhila Pullakandam, S Lakshmana Prabu, V Gopal (2010) Transdermal Drug Delivery System: An Overview. International Journal of Pharmaceutical Sciences Review and Research 3(2): 49-54.
- Md Intakhab Alam, Nawazish Alam, Vikramjit Singh, Md Sarfaraz Alam, Md Sajid Ali, et al. (2013) Type, Preparation and Evaluation of Transdermal Patch: A Review. World Journal of Pharmacy and Pharmaceutical sciences 2(4): 2199-2233.
- Archana K Gaikwad (2013) Reviewed Article, Transdermal Drug Delivery System: Formulation aspects and evaluation. Comprehensive Journal of Pharmaceutical Sciences 1(1): 1-10.
- Ajay Sharma, Seema Saini, AC Rana, Transdermal Drug Delivery System: A Review. International Journal of Research in Pharmaceutical and Biomedical Sciences.
- Nikhil Sharma, Geeta Agarwal, AC Rana, Zulfiqar Ali Bhat, Dinesh Kumar (2011) A Review, Transdermal Drug Delivery System: A Tool For Novel Drug Delivery System. International Journal of Research 3(3).
- Vinod KR, Sravani P, David Banji, Teja BB, Transdermal Drug Delivery System - Overcoming Challenges of Popular Drug Delivery Systems. International journal of Pharma world research.
- Dhiman Sonia (2011) Transdermal Patches: A Recent Approch To New Drug Delivery System. International Journal of Pharmacy and Pharm 3(5).






























