Carbon Nanotubes-Properties and Applications
Sonia Khanna1* and Nazmul Islam2
1 Department of Chemistry, School of Basic Science and Research, Sharda University, Greater Noida, India
2 Theoretical and Computational Chemistry Research Laboratory, Ramgarh Engineering College, Ramgarh, Jharkhand, India
Submission: May 19, 2018; Published: June 05, 2018
*Corresponding author: Theoretical and Computational Chemistry Research Laboratory, Ramgarh Engineering College, Ramgarh, Jharkhand, India, Email: nazmul.islam786@gmail.com
How to cite this article: Sonia K, Nazmul I. Carbon Nanotubes-Properties and Applications. Organic & Medicinal Chem IJ. 2018; 7(1): 555705. DOI 10.19080/OMCIJ.2018.07.555705
Abstract
Carbon nanotubes have gained attention in recent times due to their extraordinary physicochemical properties like strength, flexibility, sensors, conducting etc. Several preparation methods of Carbon nanotubes are discussed in this chapter. The properties and applications of nanotubes are also discussed.
Keywords:carbon nanotubes, single walled nanotubes, multiwalled nanotubes, Functionalization, nanoelectronics
Introduction
ICarbon nanotubes (CNT) are the basis of nanotechnology. Carbon with an atomic number of 6 plays a pivotal role in nanotechnology. They were discovered by Iijima [1] accidentally while studying the surface of graphite electrode used in electric arc discharge. This accidental observation laid a foundation of exciting field of nanotechnology and started a new direction in the carbon research.
A carbon nanotube (CNT) is a hexagonal array of carbon atoms rolled up into a long, thin, hollow cylinder [2] and are known for their size, shape, and remarkable physical properties. They can be manipulated chemically and physically for their application in material science, electronics, energy management, biomedical application and many more..
Structure of Nanotubes
A carbon nanotube is a tube shaped material, made of carbon and has diameter on the nanometer scale. Two types of carbon nanotubes are observed-Single walled nanotubes (SWNT) and Multiwalled nanotubes (MWNT).
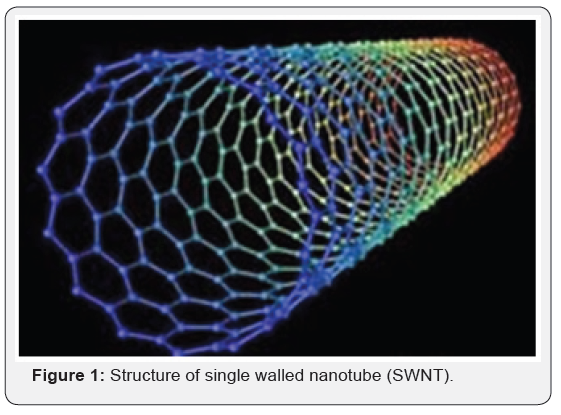
SWNT: They have diameter close to 1 nanometer and tube length may vary millions of times longer. They can be formed in three different forms – archair, zigzag and chiral forms on basis of rolling of grapheme sheet in to a seamless cylinder (Figure 1). Each form has a special effect on electrical property of nanotube.
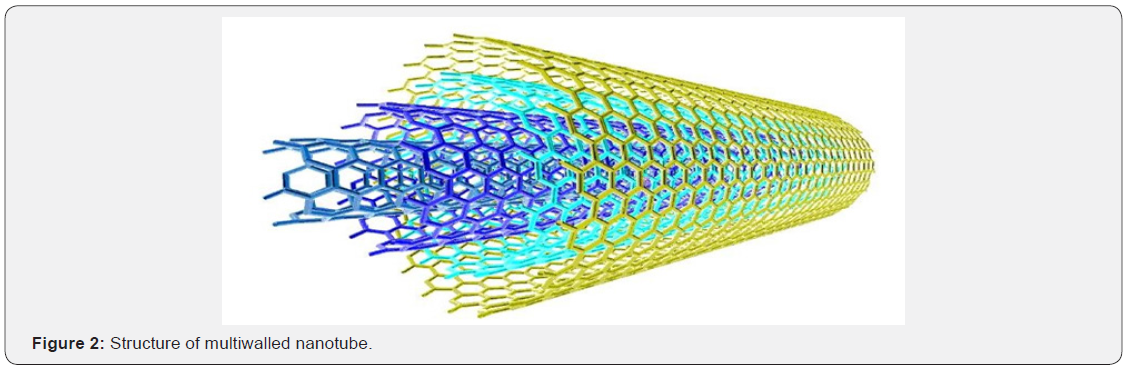
MWNT: The discovery of C60 motivated the researchers to search for other carbon compounds containing curved graphenes. This led to the discovery of multiwalled nanotubes were made of concentric cylinders of rolled-up graphene sheets, capped with semi-fullerenes. The length of a tube was in the range of a few μm, the diameter was 10-20 nm (Figure 2). As the size increases, these structures exhibit properties between fullerenes and graphite.
Two models can be used to describe the structure of multiwalled nanotubes (Figure 3). In Russian doll model, sheets of graphite are arranged in concentric cylinders within a larger single walled nanotube. In the parchment model, single sheet of graphite is rolled in around itself, resembling a scroll of parchment or rolled newspaper. The interlayer distance in multiwalled nanotubes is close to distance between graphene layers approximately 3.3Å. They have greater tensile strength than single walled nanotubes.

Synthesis of Nanotubes
There are different preparation methods for carbon nanotubes like arc discharge (AD), laser ablation, chemical vapour deposition (CVD) as well as some of the more recent methods working with high pressure of the carbon monoxide or some unique catalytic mixture. Carbon nanostructures like fullerenes, graphene and nanotubes are of great interest for the current research as well as for future industrial applications. The reason for this is that the band gap of single-walled carbon nanotubes (SWNTs) can vary from zero to about 2 eV and hence their electrical conductivity can be the one of a metal or the semiconductor.
Arc Discharge: In 1990, Kratschmer and Huffman developed an arc discharge technique and reported the synthesis of new form of solid, consisting of somewhat disordered hexagonal packing of soccer-ball shaped C60 molecule. It produces the best quality nanotubes [3-5]. During arc discharge method, a current of about 50 amps is passed between two graphite rods placed in an enclosure filled with some inert gas (like helium or argon) at low pressure (between 50 and 700 mbar). The carbon rods act as electrodes which are kept at different potentials. The anode is moved close to the cathode until an arc appears and the electrodes are kept at the distance of 1 mm for the whole duration of the process that takes about one minute. After the de-pressurisation and cooling of the chamber the nanotubes together with the by-products, can be collected. Most nanotubes deposit on the cathode. Single walled nanotubes are produced when Co and Ni or some other metal is added to the anode (Figure 4).
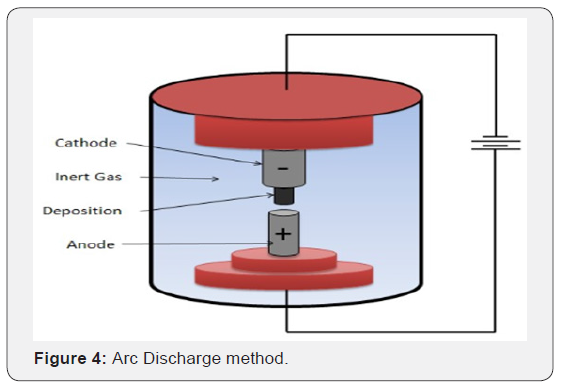
One can tune the process to produce primarily multiwalled CNTs using two standard graphite electrodes to a process that produces single-walled CNTs using metal catalysts such as Ni, Fe, Mo, or Co doped into the electrode. These metals catalyze the breakdown of gaseous molecule into carbon anf then tube starts to grow with a metal particle at the tip [6,7]. Arc discharge is the most straightforward approach to synthesize carbon nanotubes, but its application as a large-scale production technique suffers due to the moderate yield of CNTs. Ebbensen Ajayan reported large scale of MWNT by variant of standard arc discharge technique [8].
Laser Abalation: In 1995 Richard E. Smalley and his group used for the first time laser ablation to grow high quality nanotubes. Intense laser pulses ablate a carbon target which is placed in a tube-furnace heated to 1200°C. During the process some inert gas like helium or argon flows through the chamber to carry the grown nanotubes to the copper collector. After the cooling of the chamber the nanotubes and the by-products, like fullerenes and amorphous carbon over-coating on the sidewalls of nanotubes can be collected (Figure 5). The SWNT formed bundle together by van der waals forces.
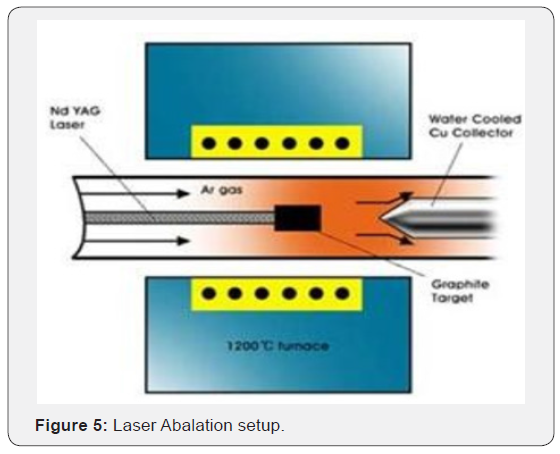
Laser ablation processes can also be tuned to yield either single-walled CNTs or multi-walled CNTs based on the variation of parameters in the growth process such as the laser wavelength, laser power, laser pulse duration, furnace temperature, and graphite target composition. In particular, a low metal : graphite ratio in the target and a high furnace temperature (typically ≈ 1200 ◦C) is generally associated with good quality crystalline single-walled CNTs, whereas greater amounts of metal yields multi-walled CNTs, and lower furnace temperatures compromise crystallinity of the CNT tube walls.
Chemical Vapour Deposition: During CVD, a substrate covered with metal catalysts, like nickel, cobalt, iron, or a combination is heated to approximately 700°C and promotes the growth of carbon nanotubes is by exciting carbon atoms that are in contact with metallic catalyst particles (Figure 6).
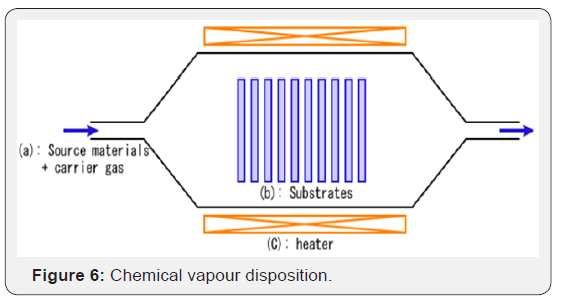
The CVD method extends this idea by embedding these metallic particles (iron, in the case of the seminal paper) in properly aligned holes in a substrate (silicon, in this case). The growth starts after two gases are passed through the chamber, a carrier gas like nitrogen, hydrogen or argon, and some hydrocarbon gas like acetylene (C2H2) or methane (CH4). The synthesis production yield, which indicates the amount of carbon nanotubes in the converted carbon, reaches 90%. CVD is commonly used for the industrial purposes because the method is already well investigated and offers acceptable results on the industrial-scale.
Size and Textural Studies of Carbon Nanomaterials
The size of the nanoparticles is a very important parameter as there is an optimal size for each application. For example, for in vivo experiments, it must be taken into account that to cross the blood brain barrier, the nanoparticles have to be in a range of 15-50 nm whereas to pass through the endothelium, they must be smaller than 150 nm. Thus, particles between 30 to 150 nm are retained in the heart, stomach and kidney whereas particles between 150-300 usually stay in liver and spleen. Another example is when magnetic particles are used as carriers for the purification of biomolecules. In this case, sizes above 40 nm are necessary in order to have a good migration toward the magnet
Functionalization of Carbon Nanotubes
Carbon nanotubes do not disperse in organic matrices due their inert nature and forms bundles with each other. The poor solubility of carbon nanotubes in organic solvents restricts them to be used as drug delivery agents into living systems in drug therapy. Hence many modification approaches like physical, chemical or combined have been exploited for their homogeneous dispersion in common solvents to improve their solubility. The surface of nanotubes can be modified by various ways to enhance their dispersion in organic media. Many applications require covalent modification to meet specific requirements i.e. in case of biosensors the biomolecules require electron mediators to promote electron transfer [9,10].
Similarly electrochemical metal ion sensors require specific functional groups which show potential affinity towards particular metal ion. The modification protocol was generally achieved by attaching specific molecule or entity which imparts chemical specificity to the substrate material. These chemical modifications can be easily achieved in many ways.
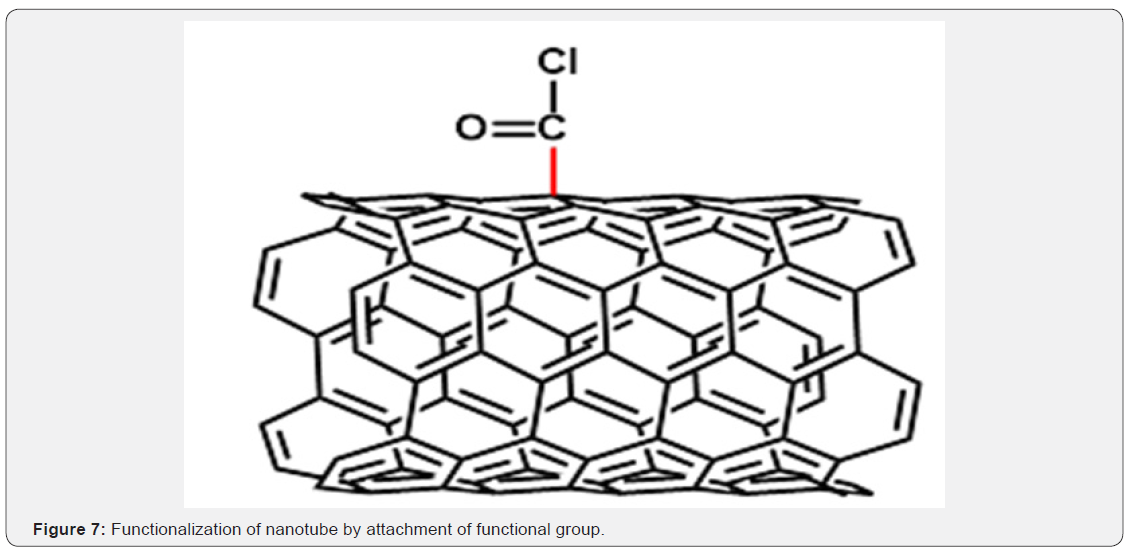
Covalent Interactions: Covalent modification involves attachment of a functional group onto the carbon nanotube. The functional groups can be attached onto the side wall or ends of the carbon nanotube. The end caps of the carbon nanotubes have the highest reactivity due to its higher pyrimidization angle and the walls of the carbon nanotubes have lower pyrimidization angles which has lower reactivity (Figure 7). Although covalent modifications are very stable, the bonding process disrupts the sp2 hybridization of the carbon atoms because a σ-bond is formed. The disruption of the extended sp2 hybridization typically decreases the conductance of the carbon nanotubes [11-13].
Oxidation: Oxidation of carbon nanotubes also functionalizes the surface by breaking the carbon-carbon bonded network of the nano layers under acidic conditions. It allows the introduction of oxygen units in the form of carboxyl, phenolic and lactone groups. In liquid-phase reactions, carbon nanotubes are treated with oxidizing solutions of nitric acid or a combination of nitric and sulphuric acid to the same effect. However, over oxidation may occur causing the carbon nanotube to break up into fragments, which are known as carbonaceous fragments. Bifeng Pan et al. [14] reported the MWCNT nano hybrids prepared initially by the oxidative pretreatment of CNTs with 3:1 H2SO4/HNO3 mixture then they were activated using SOCl2 and finally acyl chloride was coupled with ethylene diamine. The resulted MWCNTs were again modified with mercaptoacetic acid coated QDs.
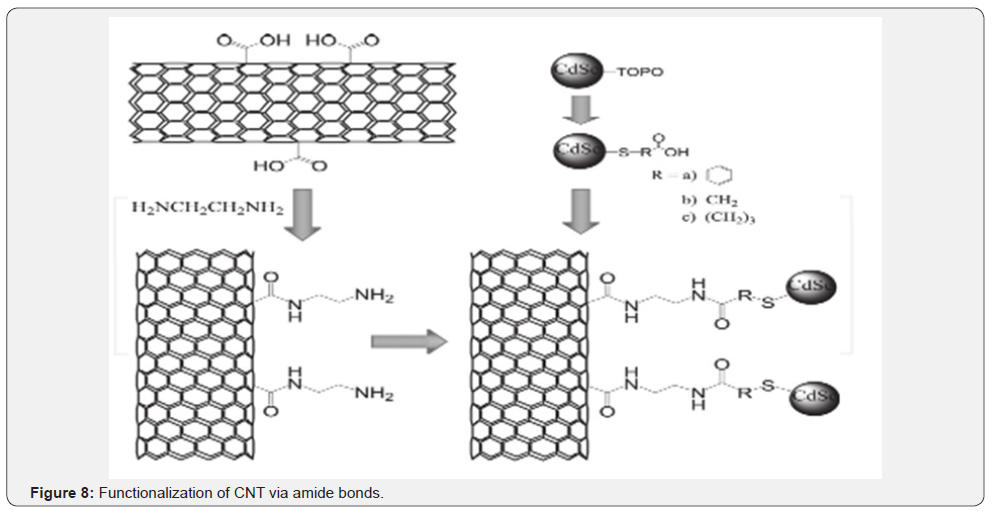
Esterification/Amidification: The functionlization of nanotubes are carried out by using carboxylic groups, which acts the precursor for most esterificationa nd amidation reactions. The carboxylic group is converted into an acyl chloride with the use of thionyl or oxalyl chloride which is then reacted with the desired amide, amine, or alcohol. . Carbon nanotubes modified with acyl chloride react readily with highly branched molecules such as poly(amindoamine), which acts as a template for silver ion and later being reduced by formaldehyde. Amino-modified carbon nanotubes can be prepared by reacting ethylenediamine with an acyl chloride functionalized carbon nanotubes. In a similar way, thiol stabilized ZnS capped CdSe QDs were protected with 2-aminoethanethiol and linked to the acid terminated carbon nanotubes in presence of a coupling agent like EDC (Figure 8) [15].
Properties of Carbon Nanotubes
Strength: Carbon nanotubes have a higher tensile strength than steel and Kevlar. This strength originates from sp2 bonds between the individual carbon atoms. Carbon nanotubes are not only strong, they are also elastic. Upon application of force, nanotube can bend and returns to its original shape when the force is removed. A nanotube’s elasticity does have a limit, and under very strong forces, it is possible to permanently deform to shape of a nanotube. A nanotube’s strength can be weakened by defects in the structure of the nanotube. Defects occur from atomic vacancies or a rearrangement of the carbon bonds. Defects in the structure can cause a small segment of the nanotube to become weaker, which in turn causes the tensile strength of the entire nanotube to weaken. The tensile strength of a nanotube depends on the strength of the weakest segment in the tube similar to the way the strength of a chain.
Electrical Properties: The sp2 bonds between carbon atoms results in conducting nature of carbon nanotubes. They can also withstand strong electric currents because of the strong nature of bonds. Single walled nanotubes can route electrical signals at speeds up to 10 GHz when used as interconnects on semi-conducting devices. Their electronic properties can be manipulatedby application of external magnetic field, mechanical force etc.
Thermal Stability: Carbon nanotubes are able to withstand high temperatures, thus acting as very good thermal conductors. The temperature stability of carbon nanotubes is estimated to be upto 28000oC and about 750oC in air. The carbon nanotubes are shown to transmit over 15 times the amount of watt per minute as compared to copper wires [16].
Applications of Carbon Nanotubes
There are many potential applications of carbon nanotubes owing to its remarkable properties. They have potential to be used in electronics, textile industry as water proof and tear proof fabric, sensor based on the property of thermal conductivity and many more. They possess extraordinary heat and electrical conductivity behaviour, making it a suitable candidate for numerous applications. . Some of the important applications of carbon nanotubes are discussed below.
Sensors: Carbon nanotubes have been reported to a good gas sensor, because of its elongated shape. They have reported to an excellent oxygen gas sensor by Zettl et al. [17]. They measured DC electrical resistance and the thermoelectric power of bundles and thin films of SWNTs. The sensitivity of carbon nanotubes to NO2 and NH3 is also reported by Dai and co-workers [18], by measuring measuring conductivity of MWNT upon exposure to NO2 and NH3 at variable time periods and temperature. Functionalised MWNT have also been used for gas sensing. Robert Haddon and colleagues found that functionalized SWNTs experienced a much greater change of resistance upon exposure to NH3 than did pristine tubes, giving them greater sensitivity as sensors. Alexander Star and colleagues from the University of Pittsburgh described a sensor for nitric oxide that employed SWNTs functionalizedwith poly (ethylene imine) [19]
Nanoelectronics: One of the potential application of nanotubes is the field of electronics, due to its highly conducting nature. Out of two types, single walled nanotubes are the most conducting type. Twisting and bending of nanotube makes it highly conducting. with high conductivity and small size, nanotubes may be an alternate option to copper which is generally used, but has limitation of ineffective at size less than 40 nm [20].
Conclusion
Carbon nanotubes have been extensively studied making way for basic understanding and potential for various applications. This article has discussed several applications of carbon nanotubes. The remarkable physical properties of carbon nanotubes have created a host of application possibilities, based on electronic and mechanical behaviour of nanotubes.
References
- Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354(56-58).
- Masciangioli T, W-X Zhang (2003) Environmental Technologies at the nanoscale. Environ Sci Technol 37(5): 102A-108A.
- J Kong, MG Chapline, HJ Dai (2001) Adv Mater 13: 1384-1386;
- XR Ye, YH Lin, CM Wang, MH Engelhard, Y Wang, et al. (2004) J Mater Chem 14: 908-913.
- Krätschmer W, Lamb LD, Fostiropoulos K, Huffman DR (1990) Solid C60: A new form of carbon. Nature 347: 354-358.
- Brenner D (1990) Emperical potential for hydrocarbons for use in simulating the chemical vapour deposition of diamond films. Physical Review B 42(15): 9458-9471.
- Calvert P (1992) Strength in unity. Nature 357: 365-366.
- Ebbesen TW, PM Ajayan (1992) large scale synthesis of carbon nanotubes Nature 358: 220-222.
- S Sampath O, Lev J (1998) Electrochemical oxidation of NADH on solgel derived, surface renewable, non-modified and mediator modified composite-carbon electrodes. Electroanal Chem 446: 57-65.
- Rico E Mojica, M Vidal, AB Pelegrina JRL (2007) Micor Voltammetric determination of lead(II) ions at carbon paste electrode modified with banana tissue. J App Sci 7(9): 1286-1292.
- Hou XM, Wang LX, Zhou F, Li LQ, LiZ (2009) Mater Lett 63: 697.
- Wu B, HuD, Kuang Y, Liu B, Zhang X, et al. (2009) Functionalization of Carbon Nanotubes by an Ionic-Liquid Polymer: Dispersion of Pt and PtRu Nanoparticles on Carbon Nanotubes and Their Electrocatalytic Oxidation of Methanol. Angew Chem Int Ed 48(26): 4751-4754.
- SJ Guo, SJ Dong, EK Wang (2010) Constructing Carbon Nanotube/Pt Nanoparticle Hybrids Using an Imidazolium-Salt-Based Ionic Liquid as a Linker. Adv Mater 22(11): 1269-1272.
- B Pan, D Cui, R He, F Gao, Y Zhang (2006) Covalent attachment of quantum dots on carbon nanotubes. Chem Phy Letters 417(4-6): 419- 424.
- Banerjee S, Wong SS (2002) Synthesis and Characterization of Carbon Nanotube-Nanocrystal Heterostructures. Nano Lett 2(3): 195-200.
- Collins PG, Bradley K, Ishigami M (2000) Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 287(5459): 1801- 1804.
- Dai HJ, Hafner JH, Rinzler AG (1996) Nanotubes as nanoprobes in scanning probe microscopy. Nature 384: 147-150.
- Kuzmych O, Allen BL, Star A (2007) Carbon nanotube sensors for exhaled breath components. Nanotechnology 18(37): 375502.
- Carbon-Nanotube Wiring Gets Real. IEEE - Spectrum 04.08.
- http://blogs.spectrum.ieee.org/articles/2008/03/carbonnanotube_ wiring_gets_rea.html






























