Synthesis New Amid of Carboxylic Acids and Antimicrobial Activity – I
Kaana Asemave1*, Andrew J Hunt2, Thomas J Farmer2 and James H Clark2
1 Department of Chemistry, Benue State University, Makurdi - Nigeria
2 University of York, United Kingdom
Submission: May 12, 2018; Published: May 30, 2018
*Corresponding author: Kaana Asemave, Department of Chemistry, Benue State University, Makurdi-Nigeria, Email: kasemave@gmail.com
How to cite this article: Kaana A, Andrew J H, Thomas J F, James H C. Modification of Biobased Lipophilic Β-Diketone. Organic & Medicinal Chem IJ. 2018; 7(1): 555703. DOI: 10.19080/OMCIJ.2018.07.555703
Abstract
A biobased lipophilic β-diketone (14, 16-hentriacontanedione, Htd) was isolated from wheat straw wax. The Htd was then modified under KF/alumina mediated Michael addition reaction in a solvent less condition with methyl acrylate and dimethyl itaconate using microwave and conventional heating. Methyl acrylate formed single and double addition with the β-diketone, while the dimethyl itaconate formed only single addition. The optimal temperature for this reaction was 120 OC. The modification was also dependent on the amount of KF/alumina used and the time of reaction. The modification using microwave and traditional heating showed marginal differences, with a slight preference towards the microwave process. The recovery yield for the methyl acrylate modified β-diketone (Ma) of 60% was higher than the 20% recovery yield of dimethyl itaconate modified β-diketone (Ita). These biobased chemicals could find use as Green metals chelators and surfactants.
Introduction
In order to ensure metals sustainability, recovery of metals from aqueous systems has been extensively explored. Therefore, many metals chelating agents such as β-diketones have been used to this effect. However, modified β-diketones with carboxylate and amine groups could act as better metals chelation agents than the unmodified ones. Ordinarily, the modification of β-diketones such as acetyl acetone and dibenzoylmethane with saturated and unsaturated organic halide has long been reported [1-3]. The alkylation of acetyl acetone with isopropyl alcohol in the presence of boron trifluoride has also been previously carried out [4] Ferrari et al. [5] modified acetyl acetone with tert-butyl bromoacetate using sodium hydride in THF at room temperature for about 24 h to form tert-Butyl-3-acetyl-4-oxopentanoate [5]. As it is, little information has been published on the modification of lipophilic β-diketones such as 14, 16-hentriacontantanedione.
Unsaturated (α,β) - carbonyl esters can be added to α-position of β-diketones using the Michael addition reaction and then hydrolyse the Michael product into acid carboxylate for enhancing the chelation ability of the β-diketone. These modified β-diketones can also be applied as surface active agents. This concept has also been similarly suggested by Fanou et al. [6]. Importantly, Michael addition is a favorable reaction from a green chemistry perspective due to its 100% atom economy. In addition, nowadays microwave heating is also efficiently used to perform Michael addition reactions and many other chemical transformations. Michael addition reactions are often carried out in polar solvents such as MeOH, acetone, DMSO, THF, [5] MeCN [7]. But, neat conditions of Michael addition reactions have been reported. This would make the reaction environmentally benign as it avoids the generation of toxic substances, like organic solvents and mineral acids.
Solvent-free microwave mediated Michael addition was also performed by Rao and Jothilingam [8]. They found that the solvent-free condition was better than using polar aprotic solvent such as DMSO [8]. Several solid heterogeneous bases including KF/alumina have find use as excellent catalysts for effecting Michael addition reactions [9,10]. These catalysts, particularly KF/alumina has attracted widespread interest because it is convenient and environmentally-benign [11,12]. Importantly, this catalyst has been effective. Wheat straw wax contain high amount fatty β-diketones which may be isolated with a green technique like scCO2 or by crashing out the β-diketones with Cu(OAc)2 from the wax solution in petroleum ether (60 - 80 OC) [13]. Therefore, this paper reports green modification of lipophilic biobased β-diketone with methyl acrylate and dimethyl itaconate.
Materials and Methods
Materials/Reagents
Cyclohexane, ethyl acetate, HCl, and petroleum ether (60-80 OC) were purchased from Fisher Scientific UK Limited. TLC plates (Merck), wheat straw wax was extracted with sc-CO2.CDCl3, methyl acrylate, dimethyl itaconate, methyl methacrylate, KF, and alumina were purchased from Sigma- Aldrich, all with purity of 99%.
Equipment
GC-MS (Perkin Elmer Clarus 500 GC coupled with a Clarus 500 quadrupole mass spectrometer), GC-FID Agilent 7820A (Agilent 6890N, Hewlett Packard HP6890; column name, RXI- 5HT; column diameter, 30 m x 0.25 mm x 0.25 μm and column maximum temperature of 400 OC), FTIR Bruker Vertex 70, 1H-NMR and 13C-NMR (JNM - ECS 400 of field strength, 400 MHz for 1H and 100 MHz for 13C), MDSC Q 2000, scCO2 extractor (SFE-500 extractor), CEM microwave, LC - Agilent 1260 Infinity and MS - Bruker micrOTOF time of flight MS, UV lamp, Biotage Isolera Four.
Purification of hentriacontane-14,16-dione from Wheat Straw Wax with Petroleum Ether
The isolation of the Htd was carried out as previously done by Horn et al. [14] with some slight modifications as reported by Asemave et al. [15] 1H-NMR (400 MHz CDCl3) δ ppm 0.79 - 0.92 (m, 9H), 1.24 (s, 60H), 1.41 (s, 3H), 1.37 (s, 4H), 1.49 - 1.75 (m, 16H), 2.22 - 2.31 (m, 5H), 2.48 (t, j = 732 Hz, 1H), 3.53 (s, 1H), 5.46 (s, 1H).13C-NMR (101 MHz, CDCl3) δ ppm 14.19 (1 C, s), 22.77 (1 C, s), 25.83 (1 C, s), 29.32 (1 C, s), 29.44 (1 C, s), 29.55 (1 C, s), 29.69 (1 C, s), 29.73 (1 C, s), 29.76 (1 C, s), 32.00 (1 C, s), 38.50 (1 C, s), 99.13 (1 C, s), 123.71 (1 C, s), 193.28 (1 C, s), 194.66 (1 C, s). FTIR (cm-1) - 2955, 2916, 2849, 1639, 1453, 1419, 1375, 1139, 907, 786, 722, 721, 631. GC-MS molecular ion; 464 m/z; Yield 18 ±5wt%. Melting point, 53.9 OC; rf (80% cyclohexane and 20% ethyl acetate): 0.89±0.03, ESI-MS(+), 463.4506 m/z, 503.4437 m/z, 549.4853 m/z.
Preparation of the KF/Alumina
KF/alumina was prepared as previously reported by Clark et al. [16] and Lenardão et al. [12].
Modification of The Bioderivedβ-Diketone
About 0.010 g of the 14,16-hentriacontanedione was weighed into the 15 mL microwave vial; follow by addition of 50 mg KF/alumina. The heterogeneous catalyst was well dispersed (suspension) with the Htd. Then 0.014 g (about 4 mole equivalents) dimethyl itaconate or 10 μL (5 mole equivalents) methyl acrylate was added to the reaction mixture. The maximum pressure and the power in the CEM discover microwave were set at 300 psi and 300 watts respectively with a reaction time between 1- 15 min. 120 OC was found to be appropriate for the modification of the Htd. These various reactions were also conducted with traditional stirrer hot plate as a heat source using identical quantities of materials in 15 mL vial with screw cap. The reaction mixture was well stirred during the reaction time under solvent-less conditions.
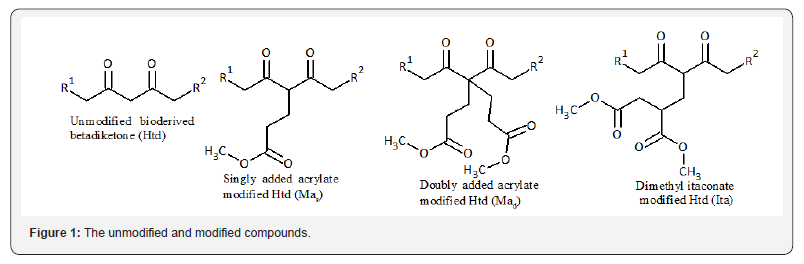
The reactions were also scaled up using 0.2 g Htd, 1 g KF/ alumina with 4 mole equivalents of the dimethyl itaconate or 5 mole equivalents of the methyl acrylate. Temperatures of 120 OC, 2 h and 60 OC, 4 h were used for the modification of the Htd with dimethyl itaconate and methyl acrylate respectively. At the end, the reaction mixtures were filtered to remove the catalyst using dichloromethane (DCM) and ethyl acetate. The products were purified using flash chromatography. In addition, short part distillation (Kugelrohr) was also applied to separate the excess dimethyl itaconate from the modified Htd. The 1H-NMR of Ma: (400 MHz, CDCl3) δ ppm 0.76 - 0.86 (6 H, m), 1.11 - 1.29 (41 H, m), 1.30 - 1.38 (3 H, m), 1.40 - 1.53 (5 H, m), 1.98 - 2.06 (3 H, m), 2.11 - 2.18 (4 H, m), 2.27 (4 H, t, J=7.32 Hz), 3.60 (6 H, s). 13C-NMR (101 MHz, CDCl3) δ ppm 14.21 (s, 1 C) 22.77 (s, 2 C) 23.67 (s, 1 C) 25.45 (s, 1 C) 28.79 (s, 1 C) 29.17 (s, 1 C) 29.37 - 29.61 (m, 4 C) 29.62 - 29.89 (m, 7 C) 31.55 (s, 1 C) 32.00 (s, 2 C) 39.29 (s, 1 C) 41.24 (s, 1 C) 42.27 (s, 1 C) 51.96 (s, 1 C) 68.77 (s, 1 C) 173.12 (s, 1 C) 193.09 (s, 1 C) 208.29 (s, 1 C). FTIR (cm-1) 2915, 2849, 1723, 1689, 1475, 1462, 1438, 1408, 1375, 1258, 1234, 1200, 1175, 1133, 1110, 1067, 985, 823, 728, 718, 653, 627. ESI-MS peaks (m/z); 675.5 (Mad), 589.5 (Mas), rf (80% cyclohexane and 20% ethyl acetate): 0.59±0.05 (Mas) and 0.41±0.05 (Mad). 1H-NMR of Ita (400 MHz, CDCl3) δ ppm 0.84 - 0.90 (5 H, m), 1.18 - 1.32 (42 H, m), 1.53 (7 H, s), 1.47 - 1.57 (3 H, m), 2.05 (2 H, s), 2.36 - 2.52 (4 H, m), 2.65 - 2.74 (2 H, m), 3.66 (2 H, s), 3.68 (2 H, s), 3.77 (1 H, dd, J=8.70, 5.50 Hz).13C-NMR (101 MHz, CDCl3) δ ppm 14.21 (s, 1 C) 23.44 (s, 1 C) 29.10 (s, 1 C) 29.22 - 29.86 (m, 10 C) 32.01 (s, 3 C) 36.41 (s, 1 C) 39.45 (s, 1 C) 41.85 (s, 1 C) 42.67 (s, 1 C) 52.01 (s, 1 C) 52.19 (s, 1 C) 65.12 (s, 1 C) 171.80 (s, 1 C) 205.54 (s, 1 C). FTIR (cm-1) 2916, 2850, 1735, 1729, 1717, 1692, 1583, 1543, 1471, 1440, 1373, 1286, 1223, 1168, 1106, 1011, 967, 888, 844, 718, 630, 544, 537, 530, 521, 515, 503. ESI-MS; (m/z) 661.5, rf (80% cyclohexane and 20% ethyl acetate): 0.51±0.05. The purified biobased products are as given in Figure 1.
Results and Discussion
Isolation of the HTD
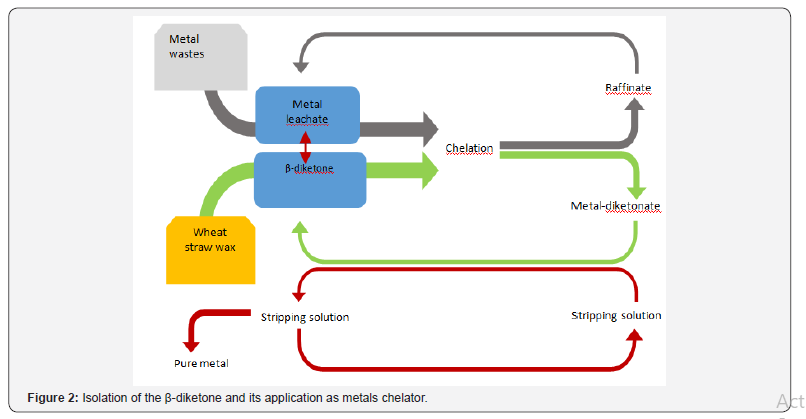
The recovered yield of the biobased β-diketone was 18±5wt%. Previous researchers have reported same high amount of β-diketone in wheat straw [17]. That means, wheat straw is a sustainable source of β-diketone which has many uses such as metals chelating agents. The following (Figure 2) describes the sustainable isolation of the β-diketone and subsequently applying it for metals extraction. This process of obtaining the lipophilic β-diketone benefit from the fact that the wax is gotten from abundant agricultural co-product, wheat straw [18] Ramaroson-Raonizafinimanana et al. [13] isolated hydrophobic β-diketone from lipids of cured and green beans by column chromatography using silica with n-hexane/diethyl ether (90:10 v/v, 200 mL). Eight fractions were obtained and the β-diketones (28%) were found in fractions 6-8 [13]. Also the purification of the hydrophobic β-diketone from this lipidic extract (2.5 g) of the beans was achieved by crashing it from 70 mL hot n-hexane with 50 mL of saturated aqueous cuprous acetate [13]. Similarly, column purification of hentriacontane-14,16-dione was performed by applying 10 g of stewart wheat wax onto a silicic acid column (400 g), where the hydrocarbons were first removed using hexane. Then esters and β-diketones (5.15 g) were eluted with hexane- CHCI3 (4: 1) [19]. The hexane and petroleum ether which are used for this purification of the lipophilic β-diketone from wheat straw wax are non-green solvents. Thus, volatile greener solvents such as 2-methyl THF and cyclohexane will be ideal for this purification of the β-diketone.
The purified β-diketone gave an ion 446 m/z due to loss of water from the molecular ion (464 m/z). The fragments with a m/z of 296 268, 281 and 309 arise from the McLafferty rearrangement at both sides of the β-diketone [17,20]. A typical fragment of 100 m/z for β-diketones was observed in this GC-MS. This ion arises as a result of a McLafferty rearrangement at the first carbonyl group which cleaves the long saturated alkyl group. Then follow by a series of keto-enol tautomerism, which result in the second carbonyl group becoming charged. This causes another McLafferty rearrangement on the second carbonyl and subsequent cleavage of another alkyl group to give rise to the peak 100 m/z. Rearrangement and loss of a methyl group from this ion gives a fragment of 85 m/z [21]. The ions 43 m/z and 57 m/z are common characteristic peaks of β-diketones as well resulting from the C-C(O) cleavage and loss of [C3H5O]+from 100 m/z fragment respectively. Therefore, the GC-MS shows that the biobased β-diketone isolated is 14,16-hentriacontanedione; which is the main abundant β-diketone found in wheat straw. The β-diketone structure was also identified using NIST library 2008.In the literature Ramaroson-Raonizafinimanana et al. [13] reported five long chain aliphatic β-diketones found in epicuticular waxes of Vanilla bean species with their molecular ions of 378 m/z (C25H46O2), 406 m/z (C27H50O2), 434 m/z (C29H54O2), 462 m/z (C31H58O2), and 490 m/z (C33H62O2) [13]. Furthermore Kenar [22] observed loss of water, [M-18]+ presumably from the enol tautomer and McLafferty rearrangements accounting for the fragment at m/z 100 in the mass spectra of long chain β-diketones. Previous studies also showed that nonacosane-10,12-dione and hentriacontane-10,12- dione were also found to form the fragment, [M-H2O]+ [23]. The ions at 43 m/z, [C2H3O]+ and 85 m/z, [C4H5O2]+ likely resulted from α-cleavage to the carbonyl carbon, whereas the ion, 57 m/z results from a cleavage of [C3H5O]+ as similarly reported [22].
Modification and Characterization of The Modified Biobased β-Diketone
Michael addition was applied for the modification of the bio derived β-diketone is described Equation 1. The Michael acceptors used were; methyl acrylate and dimethyl itaconate. Bhatt and Nimavat [24] had similarly demonstrated the preparation of 2-(2,4- dioxopentan-3-yl)benzoic acid from 2-bromo benzoic acid and acetyl acetone. In addition, Michael addition reactions are often carried out in polar solvents [7]. But here the reactions were carried out under solvent less condition as similarly reported by Ravichandran and Karthikeyan [25] in order to prevent solvent wastes, hazards, toxicity and over all making the process simpler [26,27]. Solvent-free microwave mediated Michael addition has also been performed by Rao and Jothilingam [8]. The present modification under microwave heating benefits from the fact that, ketones are medium microwave absorbers like water which can be heated efficiently [28] plus minimal by-product formation, high functional group tolerance and high conversions as it has been previously demonstrated [7]. In addition, the solvent less modification has no risk of pressure build up, hence can easily be scaled up [27]. Solvent-less and microwave are recent concept that are rampantly embraced in many chemical transformations [27,29].

Modification of the β-diketone with Michael addition reaction.
The percentage conversions under conventional heating were; 13%, 59%, 87% and for the microwave heating were; 23%, 62% and 92% with respect to methyl methacrylate, itaconate and methyl acrylate respectively. The % conversions with methyl acrylate were higher than itaconate and methyl methacrylate. At this modification condition, methyl methacrylate was least effective due to the electron donating effect of the methyl group as reported in the literature [30]. This also shows that the reaction condition was suitable in microwave irradiation as similarly performed [7,31]. Various temperatures of 333 - 453 K were tested for the modification and it was found that at 393 K, optimal conversions were obtained as 84% and 85% for dimethyl itaconate and methyl acrylate respectively. The effective conversions found at 393 K is due to the further activation of the catalyst by driving off water that might have stuck to the surface. In addition, the reaction still occurs even at lower temperature, just as it has been similarly observed [30] importantly with methyl acrylate. Going beyond this optimal temperature of 393 K for the modification will not be necessary as this will increase side reactions. It was also found that, dimethyl itaconate isomerizes at high temperature of 363 K and above in the presence of the KF/alumina at the condition of this experiment. This could also affect the conversion of the modification of the Htd with dimethyl itaconate.
From 1 to 3 min there was increase in conversions in both the microwave and conventional heating experiments. At 10 min holding time, the conversion using microwave heating reached a maximum based on this scale of the reaction unlike the conventional heating. The higher conversions in microwave heating is mostly due to the slightly longer time incurred from ramping phase. It is thought that if the overall time (including ramping period of about 3 minutes) is used in the conventional heating of the reaction, the difference in the conversions between the microwave and the conventional heating may become less noticeable. The effect of changing the amount of catalyst (KF/ alumina) on the % conversions of the modification of Htd using dimethyl itaconate and methyl acrylate was investigated. The % conversions increased faster as the amount of the catalyst increase from 15 mg through to 50 mg, but increasing the amount of the catalyst to 80 mg resulted into lower increment in the % conversions at the scale of this reaction. The modification indeed depends on the KF/alumina loading. Thus, it is reasonable from this result, to say methyl acrylate and dimethyl itaconate can be used for the modification of this Htd at 393 K or 333 K (methyl acrylate).
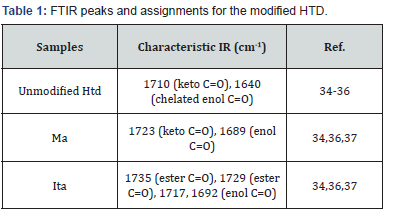
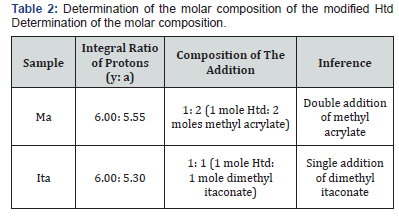
The FTIR analysis for the unmodified Htd showed absorption band at 1639 cm-1 implying the superposition of C=C and C=O stretching along with C-H bending (i.e. enol chelated form of the β-diketone) [32]. There was less intense band at 1735 cm-1 which can be assigned to keto tautomer [32,33]. The FTIR spectra of the acrylate and itaconate modified biobased β-diketones had additional carbonyl absorption due to the presence of the ester groups. The details of the FTIR absorptions are as presented in (Table 1). The 1H-NMR spectra were also used to characterize the modified Htd. The integral values of the ester protons on the modified β-diketones (a) and the six terminal protons (y) on the either sides of the saturated long chain were compared (see the structures of these products in (Figure 1). This was to check if the addition of the dimethyl itaconate and the methyl acrylate to Htd is single or double. From the integral ratios in (Table 2), it can be deduced that the addition was only single for dimethyl itaconate and a mixture of single and double with methyl acrylate. The ESI-MS (+)peaks of 589.5 m/z & 675.5 m/z; and 661.5 m/ zare also an indication of mixture of single and double addition of methyl acrylate single, and single addition of dimethyl itaconate respectively [34,35].
Chemical shift of about 3.65 ppm as it has been previously reported [36] in both 1H-NMR spectra is another confirmation of ester protons on the Ita and Ma. The 13C-NMR spectra of these modified β-diketones have the carbonyl carbon peaks of 205 - 208 ppmketo, 171 - 173 ppmester and 193 ppmenol. These chemical shifts are consistent with previous literature report for β-diketones and esters carbonyl carbons [22]. This further confirmed the products of Ma and Ita. This modification has form biobased chelating agents with zero nitrogen and phosphorus content as it is currently desired [37]. This could form yet a sustainable class of lipophilic chemicals and also help lessen the use of nitrogencontaining chelators which could cause eutrophication upon release into the environment. In addition, DSC analysis results showed that, the melting points of the Ma and Ita were found as 310 K and 319.7 K respectively. These values are lower than the melting point of the unmodified Htd (326.9 K). This may be due to the substantial H-bonding found in the unmodified Htd than in the Ma and Ita. Secondly, the Ma sample gave so much lower melting point compared to the Htd because of the sample is mixed of single and double addition products.
Conclusion
KF/alumina mediated Michael addition reaction was applied for the modification of the bio based Htd using methyl acrylate and dimethyl itaconate under microwave and conventional heating. Methyl acrylate formed single and double addition with the bio derived β-diketone, while the itaconate formed only single addition. The optimal temperature for this reaction was 120 OC. The modification was also dependent on the amount of KF/A2O3 used and the time of reaction. The modification using microwave and traditional heating showed marginal differences, with a slight preference towards the microwave process. These biobased molecules can find use as metals extract ants, surfactants and in care products.
Acknowledgement
We would like to thank the Tertiary Education Trust Fund (TET Fund) - Nigeria and Benue State University, Makurdi, Nigeria for their kind financial support and University of York where these studies were carried out.
References
- Staniszewski B, Urbaniak WW (2009) A simple and efficient synthesis of 3-substituted derivatives of pentane-2, 4-Dione. Chem Pap 63(2): 212-216.
- Tundo P, Venturello P, Angeletti E (1987) Alkylation Reactions of Ethyl Malonate, Ethyl Acetoacetate, and Acetylacetone by Gas-Liquid Phase- Transfer Catalysis (GL-PTC). J Chem Soc Perkin Trans 2159-2162.
- Kalyanam N, Karban JW, JLM (1979) The Mono alkylation of Dibenzoyl methane. Org Prep Proced Int New J Org Synth 11: 100.
- Rimmin TC, Hauser C (1967) Alkylation of Acetylacetone with Isopropyl Alcohol by Means of Boron Fluoride. J Org Chem 32(8): 2615-2616.
- Ferrari E, Saladini M, Pignedoli F, Spagnolo F, Benassi R (2011) Solvent effect on keto-enol tautomerism in a new b-diketone: a comparison between experimental data and different theoretical approaches. New J Chem 35: 2840-2847.
- Fanou D, Yao B, Siaka S, Ado G (2007) Heavy metals removal in aqueous solution by two delta-diketones. Jour Appl Sci 7(2): 310-313.
- Li GZ (2010) Investigation into thiol-(meth) acrylate Michael addition reactions using amine and phosphine catalysts. Polym Chem (RSC) 1: 1196-1204.
- Rao HSP, Jothilingam S (2005) Solvent-free microwave-mediated Michael addition reactions. J Chem Sci 117(4): 323-328.
- Girling K (2011) Heterogeneously catalysed isomerisation of allylbenzene- MSc(R) thesis. University of Glasgow, Scotland.
- Basu B, Das P, Das S (2008) Recent Advances in KF/alumina Promoted Organic Reactions. Curr Org Chem 12(2): 141-158.
- Ono Y, Baba T (1997) Selective reactions over solid base catalysts. Catal Today 38(3): 321-337.
- Lenardão EJ, Trecha DO, Ferreira P, Jacob RG, Perin G, et al. (2009) Green Michael addition of thiols to electron deficient alkenes using KF/ alumina and recyclable solvent or solvent-free conditions. J Braz Chem Soc 20(1): 93-99.
- Ramaroson-Raonizafinimanana B, Gaydou EM, Bombarda I (2000) Long-Chain Aliphatic β-Diketones from Epicuticular Wax of Vanilla Bean Species. Synthesis of Nervonoylacetone. J Agric Food Chem 48(10): 4739-4743.
- Horn DHS, Kranz ZH, Lamberton JA (1964) Austrialian J Chem 17: 464 - 476.
- Asemave K, Bryne F, Hunt AJ, Farmer TJ, Clark JH (2016) Electronic Supporting Information on Rapid and Efficient Biphasic Liquid Extraction of Metals with Bioderived Lipophilic β-diketone. RSC Adv S1-S8.
- Clark JH, Farmer TJ, Macquarrie DJ (2007) The Derivatisation of bio- Platform Molecules Using KF-Alumina Catalysis. Chem Sus Chem 2(11): 1025-1027.
- Del Río JC, Prinsen P, Gutiérrez A (2013) A comprehensive characterization of lipids in wheat straw. J Agric Food Chem 61(8): 1904-1913.
- Abushi T, Asayuki M (1959) Solvent Extraction of Iron with Chloroform as Acetylacetonate. Bull Inst Chem Res Kyoto Univ 37(4): 232-236.
- Tulloch AP, Hoffman LL (1971) Leaf Wax of Durum Wheat. Phyto chemistry 10(4): 871-876.
- Colnaghi SAV, Silva DS Da, Lambais MR, Carrilho E (2007) Characterisation of of a Putative Xylella Fastidiosa Diffusible Signal Factor by HRGC-EI-MS. J Mass Spectrom 42(4): 490-496.
- Prinsen P, Gutiérrez A, Río JC Del (2012) Lipophilic Extractives from the Cortex and Pith of Elephant Grass (Pennisetum purpureum Schumach.) Stems. J Agric Food Chem 60(25): 6408-6417.
- Kenar JA (2003) Preparation of Long-Chain β-enaminones and β-diketones from long-chain3,5-iisubstituted Isoxazole compounds. pp. 1027-1032.
- Jenks MA, Gaston CH, Goodwin MS, Keith JA, Teusink RS (2012) Seasonal Variation in Cuticular Waxes on Hosta Genotypes Differing in Leaf Surface Glaucousness. Hortscience 37(4): 673-677.
- Bhatt ND, Nimavat K (2013) Synthesis and Characterisation of Novel 3-Substituted 2,4-Pentane Diones. Int J Pharm Res Sch 2: 51-53.
- Ravichandran S, Karthikeyan E (2011) Microwave synthesis- A potential tool for green chemistry. Int J Chem Tech Res 3(1): 466-470.
- Tanaka K (2003) Solvent-free Organic Synthesis. WILEY-VCH Verlag GmbH & Co KGaA, Weinheim.
- Varma RS (1999) Solvent-free organic syntheses: using supported reagents and microwave irradiation. Green Chem 1: 43-55.
- Hayes BL (2002) In Microwave synthesis: Chemistry at the speed of Light 1-291.
- Luu TXT, Lam TT, Le TN, Duus F (2009) Fast and Green Microwave- Assisted Conversion of Essential Oil Allylbenzenes into the Corresponding Aldehydes via Alkene Isomerization and Subsequent Potassium Permanganate Promoted Oxidative Alkene Group Cleavage. Molecules 14(9): 3411-3424.
- Escalante J, Carrillo-Morales M, Linzaga I (2008) Michael Additions of Amines to Methyl Acrylates Promoted by Microwave Irradiation. Molecules 13(2): 340-347.
- Orlando US, Baes AU, Nishijima W, Okada M (2002) Preparation of chelating agents from sugarcane bagasse by microwave radiation as an alternative ecologically benign procedure. Green Chem 4: 555-557.
- Abood NA, Ajam AF (1985) Infrared study of keto-enol equilibrium of acetylacetone, benzoylacetone and dibenzoylmethane in various organic solvents. J Chem Soc Pak 7: 1-6.
- Lozada-Garcia RR, Ceponkus J, Chin W, Chevalier M, Crépin C (2011) Acetylacetone in hydrogen solids: IR signatures of the enol and keto tautomers and UV induced tautomerization. Chem Phys Lett 504(4-6): 142-147.
- Bull OS, Obunwo CC (2014) Synthesis And Spectroscopic Studies of Tetrakis(Acetato)Copper(Ii) Dihydrate Complex. Chem Mater Res 6(1): 69-75.
- Sohn JR, Lee SI (1997) An Infrared Spectroscopic Study of Acetylacetone Adsorbed on Layer Silicates Containing Various Interlayer Cations. J Ind Eng Chem 3: 198-202.
- Parsons AF (2003) Keynotes in organic chemistry. Blackwell publishing.
- Sheikh J, Juneja H, Ingle V, Ali P, Hadda T, Ben (2013) Synthesis and in vitro biology of Co(II), Ni(II), Cu(II) and Zinc(II) complexes of functionalized beta-diketone bearing energy buried potential antibacterial and antiviral O,O pharmacophore sites. J Saudi Chem Soc 17(3): 269-276.






























