Synthesis of Carbon Nano tubes by Flame Fragments Deposition of Liquefied Petroleum Gas
Asmaa H Hammadi1, Firas H Abdulrazzak2, Abbas J Atiyah3 and Falah H. Hussein4*
1,4College of Pharmacy, University of Babylon, Hilla, Iraq
2Chemistry Department, College of Education for Pure Sciences, Diyala University, Iraq
3Department of Chemistry, College of Science, Babylon University, Hilla, Iraq
Submission: May 16, 2018; Published: May 19, 2018
*Corresponding author: Falah H Hussein, College of Pharmacy, University of Babylon P.O. Box 51002, Hilla, Iraq, Email: abohasan_hilla@yahoo.com
How to cite this article: Asmaa H H, Firas H A, Abbas J A, Falah H. H. Synthesis of Carbon Nano tubes by Flame Fragments Deposition of Liquefied Petroleum Gas. Organic & Medicinal Chem IJ. 2018; 6(5): 555699. DOI: 10.19080/OMCIJ.2018.06.555699
Abstract
Carbon nanotubes (CNTs) were synthesized by flame fragments deposition (FFD) of liquefied petroleum gas (LPG). In this method liquefied petroleum gas was used as a carbon source for synthesization of CNTs and as a source for the required energy. The deposition of CNTs was done at low temperature (1600C). The liquefied petroleum gas was feeded into a homemade instrument. The Instrument consists of internal burner to burn the liquefied petroleum gas in the existence of oxygen gas to maintain the continuity of the flame of the burner while nitrogen gas is used to keep the flame at suitable form. In addition nitrogen gas is used to cool the synthesized sample. Gases flow rates are controlled by specific gauges. The synthesization was carried on without using catalyst. Synthesized product was analysed by X-ray diffraction (XRD), transmission electron microscopy (TEM), Surface area (BET), and Raman spectroscopy, thermo gravimetric analysis (TGA), Energy Dispersive X-ray Spectroscopy (EDS) and X-ray Photoelectron Spectroscopy (XPS). The results suggested the formation of carbon nanotubes with a purity exceeding 65% with an outer diameter of 6.9-9.7nm, an inner diameter of 2.75-5.88 nm, a thickness of 3-5 graphene layers and a length of about 1-2 μm.
Keywords: Flame Fragments Deposition; Carbon Nanotubes; Liquefied Petroleum Gas
Abbrevations:CNTs: Carbon Nanotubes; CVD: Chemical Vapour Deposition; SWCNTs: Single Wall Carbon Nanotubes; MWCNTs: Multi-Wall Carbon Nanotubes; FFD: Flame Fragments Deposition; XRD: X-Ray Diffraction; TEM: Transmission Electron Microscopy; BET: Surface Area; TGA: Thermo Gravimetric Analysis; EDS: Dispersive X-Ray Spectroscopy; XPS: X-Ray Photoelectron Spectroscopy
Introduction
The discovery of carbon nanotubes (CNTs) in 1991 has the potential of revolutionizing the wide range of applications in different fields due to their unique structure and extraordinary mechanical and electronic properties [1]. Four main methods are currently being employed to synthesize carbon nanotubes. These methods are: arc discharge, laser ablation and chemical vapour deposition (CVD) and autoclave processes. CVD method is the most applicable and economical method for producing quantitative and qualitative single wall carbon nanotubes (SWCNTs) or multi-wall carbon nanotubes (MWCNTs) [2-5]. Innovative approach was employed in this work, with an attempt to develop a technique that can synthesize CNTs from liquefied petroleum gas without using another energy source and without using catalysts at low temperature within short time by using a homemade instrument [6,7].
Materials and Method
Homemade Flame Fragments Deposition Instrument
The homemade flame fragments deposition instrument consists two stainless steel boxes. The internal box with dimensions 41×38×25cm. Liquefied petroleum gas, oxygen and nitrogen gases interned the system through three inlet stainless tubes with a diameter of 1.5 cm. The flow rate of gases was controlled by outside gauges. The instrument consisted a gas gate for the excess gases. On the top of the box there are nine positions fitted to hold the crucibles which used as sample collectors. These collectors are in touch with upper cold lid. The outer box with dimensions 50×47×38cm. The outer box has an airtight cover. The instrument has a front lens with dimensions 14x13cm used to monitor the intensity of the flame. The schematic diagram of homemade flame fragments deposition instrument is shown in Figure 1. Iraqi liquefied petroleum gas used as sources for carbon, oxygen gas was used in order to provide a natural combustion atmosphere for liquefied petroleum gas and nitrogen gas was used as inert gas and this gas helps to control the nature of combustion of liquefied petroleum gas. Nitrogen gas also used to cool the instrument at the end of each experiment. The process synthesis nanotubes in this way require combustion to be incomplete for liquefied petroleum gas. After the gases entered the furnace, the combustion occurred, a yellow flame was formed, after which the nanotube deposition occurs on the surface of each crucible. The time required for complete process is 30 min. The inside temperature in homemade instrument is 140-150 ºC.

Results and Discussion
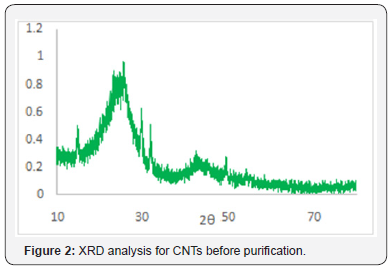
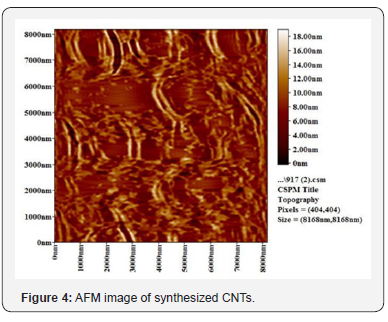
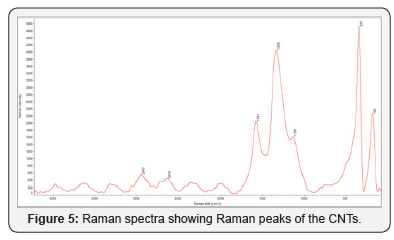
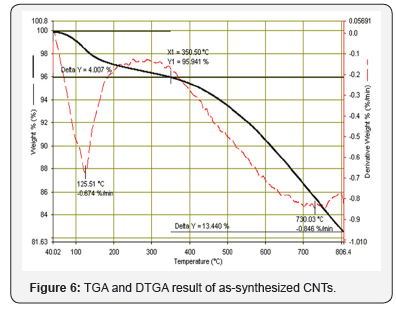
Figure 2 shows the XRD of synthesized carbon nanotubes which clearly indicates the graphitic peaks related to the (002) which called Bragg peaks and the position of this peak located at 28o and this is due to the presence of nanotube structure of carbon atoms, this means that the synthesized sample has nanotubes structures. Other peaks related to the in-plane (100) near 43o of nanotube structure [8]. Figures 3 & 4 show SEM and AFM morphological images of CNTs. The CNTs consist of SWCNTs and MWCNTs which we could confirm due to the presence of RBM in Raman spectrum [9] (Figure 5). CNTs diameters are in the range of 26.91-31.62nm. The CNTs are elongated and measured about 2μm. and the ratio of nanoparticle and nanotube is 82%. Based on the EDX analysis and TGA result, as shown in Figure 6, the synthesized CNTs sample needs more purification. 12% of the sample contains moisture and organic compounds which was clear before further purification. The removal of organic materials from the synthesized sample by addition of acetone improved the properties of synthesized CNTs

Conclusion
This study offers an economical and an alternative technique of synthesizing CNTs at low temperature in the absence of any types of catalysts which are mostly increase the impurities of synthesized CNTs and without need for sophisticated tools as that used in classical chemical vapour deposition (CVD) technique. A mixture of SWCNTs and MWCNTs was obtained, similar to most CVD techniques.
References
- S Iijima (1991) Helical microtubules of graphitic carbon. Nature 354: 56-58.
- M Bierdel, S Buchholz, V Michele, L Mleczko, R Rudolf, et al. (2007) physica status solidi (b) 244 3939.
- K Hernadi, Z Konya, A Siska, J Kiss, A Oszko, et al. (2002) Materials Chemistry and Physics 77536.
- Z Li, E Dervishi, Y Xu, X Ma, V Saini, et al. (2008) Effects of the Fe-Co interaction on the growth of multiwall carbon nanotubes. Journal of Chemisty and Physics 129(7).
- A Magrez, JW Seo, C Miko, K Hernadi, L Forro (2005) Journal of Physical Chemistry B 109: 10087.
- FH Hussein FH, Abdalrazak, Alkaim A (2017) Patent COSQC, IRAQ.
- AM Jassm, FH Hussein, FH Abdulrazzak, AF Alkaim, BA Joda (2017) Asian J Chem 29(12) 2804.
- Y Peng, H Liu (2006) Effects of oxidation by hydrogen peroxide on the structures of multiwalled carbon nanotubes. Ind Eng Chem Res 45(19): 6483-6488.
- MS Dresselhaus, G Dresselhaus, A Jorio, AG Souza Filho, et al. (2002) Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 40(12): 2043-2061.






























