DSC Thermal Analysis for Some Calcium Iron Arsi- Vanadate Oxide Glasses
AG Mostafa1, MY Hassan1 and HM Goumaa2*
1 Physics Department, Faculty of Science, AL-Zahra University, Nasr City, Cairo, EgYPT
2 Optical Branch, High Institute of Optics Technology, Cairo, Egypt
Submission: May 01, 2018; Published: May 14, 2018
*Corresponding author: HM Goumaa, Optical Branch, High Institute of Optics Technology, Cairo, Egypt, Email: h_goumaa@yahoo.com
How to cite this article: AG Mostafa, MY Hassan, HM Goumaa. DSC Thermal Analysis for Some Calcium Iron Arsi- Vanadate Oxide Glasses. Organic & 002 Medicinal Chem IJ. 2018; 6(4): 555693. DOI: 10.19080/OMCIJ.2018.06.555693
Abstract
Glasses with molar composition of (55 - x) molpercentage As2O3 - (25 + x) molpercentage V2O5 - 10 molpercentage Fe2O3 - 10 molpercentage CaO, where x = 0, 5, 10, 15, 20, 25 and 30, have been prepared using the normal melt quench method. The effect of replacement As2O3 by V2O5 on the structure of these glasses have been investigated by Differential scanning calorimetric DSC thermal analysis, Density d measurement and Molar volume Vm calculation. For each sample, DSC showed the presence of a single glass transition temperature Tg in addition to two crystallization peak s. The activation energy for glass transition indicates that the prepared glasses may be of high stability. Both the density and the molar volume changed oppositely with change in composition, indicating significant change of the structural units.
Keywords: Oxide glass; DSC-Glass Transition Temperature; Iron doped glasses; Vanadate glasses; Structure
Introduction
In recent years glasses biased on V2O5 has attracted attention because of its potential use as cathode in solid-state devices. These glasses electrical properties classified to be similar to the n-type semiconductor. Also it was found that their electronic conduction is caused by a phonon assisted electron hopping between V4+ and V5+ ions [1-2]. Studies, on glasses containing relatively low concentration of V2O5, show that these glasses have a potential application as optical and electrical memory switching, cathode materials for making solid-state devices and optical fiber [1-3]. In the glasses with high percentage of V2O5, it is considered as a glass forming oxide [3-4]. Since the arsenic oxide As2O3 is consider as one of the most efficient fining agent for glass melt because of its ability for removing bubbles from glass mass [5], this article aims to characterize the glassy state of (25+x) mole% V2O5- (55-x) mole% As2O3 -10 mole% Fe2O3-10 mole% CaO glass system.
Experimental Work
(55 - x) mol% As2O3- (25 + x) mol% V2O5-10 mol% Fe2O3- 10 mol% CaO, glass system have been prepared, by the melt quenching, on the bases of the percentage molecular weights, as shown in Table 1. The start materials were of purity not less than 99.98% while V2O5, As2O3 and Fe2O3 added as such and CaO was introduced as Calcium Carbonate. The finely mixed batches were melted in porcelain crucibles in an electric muffle furnace for 2- Hour, at temperature 750±20 ºC. The melts were stirred several times during melting, and they were then poured between two pre-cooled stainless steel plates in air. The thermal measurements were carried out by using Shimadzu 50-DSC Analyzer with heating rates 5, 10, 20, 30 and 40 K/min in the temperature range (303-1000) K. the calorimetric was calibrated for each heating rate using a material of well known melting temperature and enthalpy.
Results and Discussion
DSC Thermal Analysis
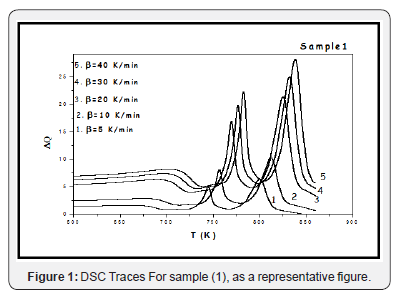
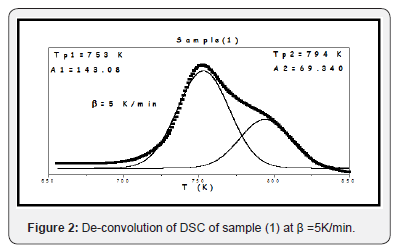
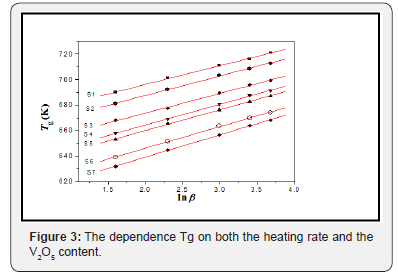
Differential scanning calorimetric DSC thermal analysis has been used in studying the thermal stability of the prepared glasses. Some parameters have been measured easily and accurately, such as the glass transition Tg and crystallization Tp temperatures, during different heating Processes with different rates. Figure 1 exhibits the DSC traces for sample [1] during different heating rates, β=5, 10, 20, 30 and 40K/min, as a representative Figure, all studied samples were showed the same shapes. For all samples, by inspecting in DSC Spectra only one glass transition temperature in addition to two overlapping exothermic crystallization peaks have been observed, for each heating rate. The crystallization peaks identified and separated by using the de-convolution method, as in Figure 2, as a representative curve, for sample (1). Two crystallization peaks appearance may be indicating that there are two different phases appearing during the crystallization process, as a result to the fact that both V2O5 and Fe2O3 act as nucleating agents [6-8]. For each sample, the appearance of one glass transition temperature may indicate high homogeneity, good glassy stare formation [9]. Table 2 exhibits the obtained thermal parameters of the studied glasses, at different heating rates. By inspecting this table, an increase in the glass transition temperature and glass crystallization temperature have been observed with the heating rate increasing. Such behavior may be indicating an increase in the thermal stability of all investigated glasses as the heating rate increase [6-10]. The dependence of the glass transition temperature (Tg) on the heating rate has been studied by applying Kissinger’s formula Ln(T2g/β) = (Eg/RTg) + constant, Figure 3 Where ΔEg is the activation energy for glass transition, R is the gas constant and β is the heating rate. ΔEg obtained from plotting ln (T2g/β) against (1/Tg) which is a straight line of a slope equal (ΔEg/R), Table 3 exhibits the obtained values of Eg for the studied glass system. Also, Figure 3 exhibits the effect of the V2O5 content increment on the glass transition temperature (Tg), which appears to decrease linearly as V2O5 content increase. Like behavior may be implies a decrease in the glass thermal stability, in other word a decrease in the rigidity of the glass network [11]. Since the unstable glasses have a loose packing, therefore it can predict an increase in the molar volume of the studied glasses as V2O5 content was increased.
Density (ρ) and Molar Volume (Vm)
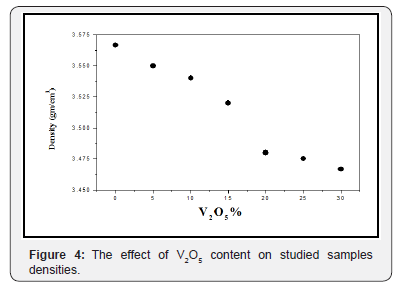
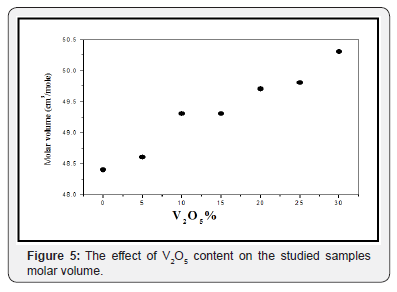
Density is an important and accurate property, which strongly reflects the fine changes in the glass structure. Therefore, the liquid displacement method used to obtain the room temperature experimental density of the studied glasses and plotted as a function of V2O5 content in Figure 4. It is clear that the measured density decreases approximately linear with As2O3 replacement by V2O5. The molar volume is directly related to the internal spatial structure of glass so it is more suitable to discuss the differences between the structures of the studied glasses in terms of the molar volume [12]. Figure 5 exhibits the calculated molar volume which appeared to increase linearly as V2O5 content was gradually increased, indicating a loose packed structure in an agreement with DSC results. The behavior of both density and molar volume can be attributed to the generally increase in the oxygen atoms, the increase in the non-bridging oxygen (V=O) atoms, in addition to the difference in the atomic weight of V5+ {50} and As3+ {74.9} cations [13]. The inversely variation of both the density and molar volume indicates significant change of the structural units with change in composition.
Conclusion
(55-x) % As2O3. (25+x)% V2O5. 10% Fe2O3. 10 %CaO, Where 0 ≤ x ≤ 30, glass system have been studied. DSC thermal analysis showed the presence of one glass transition temperature and indicated high homogeneity of the prepared glasses. DSC Also showed the presence of two crystallization peaks indicate the existence of two different crystalline phases. The values of experimental density have found to decrease as V2O5 content was increased. The calculated molar volume values showed an increase, indicating a more open structure, as V2O5 content was increased. The inversely variation was observed for both the density and molar volume indicates significant change of the structural units with change in composition.
References
- S Sindhu, S Sanghi, A Agarwal, Sonam, VP Seth (2005) The Role of V2O5 in the Modification of Structural, Optical and Electrical Properties of Vanadium Barium Borate Glasses. Physica B: Condensed Matter 365(1- 4): 65-75.
- GD Khattak, N Tabet (2004) Local structure and redox state of vanadium in strontium-vanadate glasses. J Electron Spectroscopy and Related Phenomena 36(5): 257-264.
- S Sindhu, S Sanghi, A Agarwal, VP Seth, N Kishore (2006) Structural, optical, physical and electrical properties of V2O5.SrO.B2O3 glasses. J Spectrochimica Acta Part A 64(1): 196-204.
- A Mekki, GD Khattak, D Holland, M Chinkhota, LE Wenger (2003) Structure and magnetic properties of vanadium–sodium silicate glasses. Journal of Non-Crystalline Solids 318(1-2): 193-201.
- Paulo Ccero do Nascimento, Denise Bohrer, Emilene Becker, Leandro Machado de Carvalho (2005) Comparison of different sample treatments for arsenic speciation in glass samples. J Non-Crystalline Solids 351(14-15): 1312-1316.
- ER Shaaban, MT Dessouky, AM Abousehly (2007) J Physics Condensed Mater 19: 096-212.
- ER Shaaban (2006) Non-isothermal crystallization kinetic studies on a ternary, Sb0.14As0.38Se0.48 chalcogenide semi-conducting glass. Physica B: Condensed Matter 373(2): 211-216.
- I Kashif, H Farouk, AM Sanad, SA Aly (1992) Structural studies of some V2O5-P2O5-B2O3-Fe2O3 glass systems. J Material Science 27(1): 122-126.
- BV Raghavaiah, N Veetaiah (2004) J Phys Chem of solids 65(6): 1153- 1164.
- NC De Souza, R Lebullenger, MCC Custodio, AC Hernandes (2000) J Non Crystalline Solids 273: 94-99.
- A AbdEl Moneim (2002) J Materials Chem and Phys 73: 318-322.
- V Raghavaiah, C Laxmianth, N Veeraiah (2004) J Optics Communications 235: 341-349.
- H Mori, H Matsuno, H Sakata (2000) J Non-Crystalline Solids 276: 78- 94.






























