Stereoselective Synthesis of (3S, 4R)-5-Phenylpentane-1, 3, 4-Triol
R Venkateshwarlu, S Purushotham Reddy, P Venkateswara Rao* and S Aravind*
Department of Chemistry, Osmania University, India
Submission: May 03, 2018; Published: May 09, 2018
*Corresponding author: P Venkateswara Rao and S Aravind, Department of Chemistry, Osmania University, India; Email: pallapothulav@gmail.com;aravind.iict@gmail.com
How to cite this article: R Venkateshwarlu, S P Reddy, P V Rao, S Aravind. Stereoselective Synthesis of (3S, 4R)-5-Phenylpentane-1, 3, 4-Triol. Organic & Medicinal Chem IJ. 2018; 6(4): 555691. DOI: 10.19080/OMCIJ.2018.06.555691
Abstract
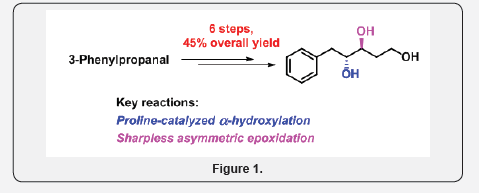
We describe an efficient synthesis of (3S, 4R)-5-Phenylpentane-1, 3, 4-triol (1) from commercially available 3-phenylpropanal for the first time. The key reactions involved in this synthesis are proline catalyzed hydroxylation, followed by (Z)-selective Wittig olefination, and Sharpless asymmetric epoxidation (Figure 1).
Keywords: Natural product; Triol; Wittig olefination; Total synthesis; Alzheimers; Parkinsons
Introduction
Polyhydroxylated compounds are ubiquitous structural motifs found in a multitude of naturally occurring compounds, pharmaceuticals and material interest [1-5]. In addition to this their synthetic analogues are important as lead structures or drug candidates for the discovery of novel drugs [6-9]. These compounds have explicitly exhibited a broad spectrum of biological activities including antibacterial, antitumoral, antimicrobial, antifeedant, herbicidal, plant growth inhibition and the inhibition of cholesterol biosynthesis properties [10-14]. Recently Hirokazu Kawagishi et al. isolated the triol compound named (3S,4R)-5-Phenylpentane-1,3,4-triol (1, Scheme 1) from the EtOH extract of edible mushroom Mycoleptodonoides aitchisonii [15]. It exhibits protective activity against endoplasmic reticulum (ER) stress-dependent cell death. ER stress is caused by abnormalities in cell function such as changes in calcium channel functioning or accumulation of misfolded protein and this may be responsible for Parkinson’s, Alzheimer’s and prion type of human neuronal diseases, and also other diseases (diabetes, atherosclerosis, and heart & liver disease) [16,17]. Therefore, development of efficient strategies for the preparation of natural and unnatural products, which exhibits protective activity against endoplasmic reticulum stress-dependent cell death, is of great significance. Due to its interesting structural features and evident pharmacological potential, the synthesis of 1 has attracted much attention for the synthetic and medicinal chemists. In continuation of our research on the synthesis of biologically significant natural products from simple starting materials [18,19]. We herein, report a first total synthesis of 1, starting from 3-phenylpropanal (2) in a six steps with 45% overall yield.
Results and Discussions
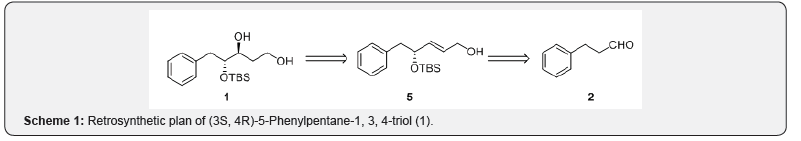
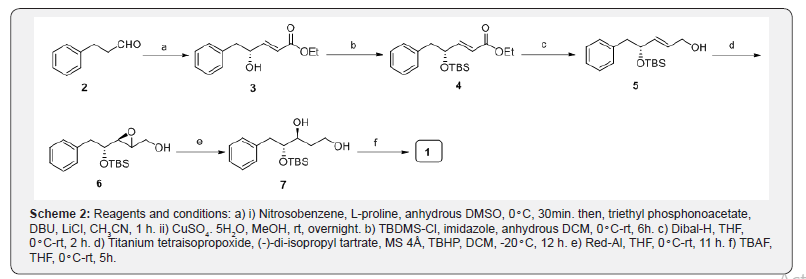
Our approach to the asymmetric synthesis of (3S, 4R)- 5-Phenylpentane-1, 3, 4-triol (1) is shown in scheme 1. We envisioned that the target molecule can be derived from allyl alcohol 5 via Sharpless asymmetric epoxidation protocol. The allylic alcohol 5 from 3-phenylpropanal (2) using proline catalyzed sequential -aminooxylation and Horner-Wadsworth- Emmons olefination. The synthetic sequence began with the preparation of ester fragment 3 from commercially available 3-phenylpropanal (2, scheme 2). Thus, phenylpropanal (2) was subjected to aminooxylation process by using nitrosobenzene as an oxygen source and L-proline as a catalyst at -20C, followed by in situ (Z)-selective Wittig olefination reaction with the triethyl phosphonoacetate, LiCl and DBU to furnish crude -aminooxy ester [20]. Subsequent reduction of the -aminooxy compound with 30 mol % CuSO4.5H2O in methanol provided the hydroxy unsaturated ester 3 in 69% yield. The enantiomeric purity of the hydroxyl ester 3 was determined as 99% by using chiral HPLC analysis. The protection of the hydroxy group in compound 3 with TBS-Cl, imidazole in THF gave silyl ether compound 4 in 96% yield [21]. The reduction of ester functionality in compound 4 was carried out with Dibal-H in THF at room temperature to afford allylic alcohol 5 in 92% yield [22]. Next, installation of chiral epoxide on intermediate 5, has been achieved using Sharpless asymmetric epoxidation protocol with (-)-di-isopropyl tartrate, tert-butyl hydroperaxide and titanium tetra (isopropaxide) in tert-butanol and water at -20C for 12h, gave the chiral epoxide 6 in 91% yield [23]. Opening of the epoxide in a compound 6 with Red-Al provided diol 7 in 86% yield [24]. Finally, deprotection of the silyl group in 1, 3-diol 7 with TBAF in THF yielded the title compound, (3S, 4R)-5-Phenylpentane-1, 3, 4-triol (1) in 95% yield.
In conclusion, the first asymmetric synthesis of (3S, 4R)- 5-phenylpentane-1, 3, 4-triol (1) starting from commercially available 2-phenylpropioal has been achieved in a six steps with 45% overall yield. The key reactions include, a proline-catalyzed -aminooxylation, followed by (Z)-selective Wittig olefination and Sharpless asymmetric epoxidation.
Experimental
Synthesis of ((3S)-3-((R)-1-((tert-butyldimethylsilyl) oxy)-2-phenylethyl)oxiran-2-yl)methanol (6)
To a stirred mixture of powdered molecular sieves (4Å, 4.0g) and titanium tetraisopropoxide (1.37mL, 1.37mmol, 1M solution in CH2Cl2, 0.2equiv.) in (20mL) cooled at -20°C was added a (-)-di-isopropyl tartrate in CH2Cl2 (0.64g, 2.74mmol, 0.4equiv.). The mixture was stirred at -20°C for 10min, and a solution of allylic alcohol 5(2.00g, 6.84mmol, 1equiv.) in CH2Cl2(15mL) and a 5M solution (1,2-dichloroethane) of tert-butylhydroperoxide (2.46g, 5.47mL, 27.40mmol, 4equiv.) were added, successively. The resulting mixture was stirred at -20°C for 12h and quenched with 3mL H2O and 0.8ml 20% NaOH. After the mixture was stirred at rt for 45min, the organic layer was separated, and filtered the reaction mixture. The aqueous layer was extracted with CH2Cl2 (2 x 30mL). The organic layer and the extracts were combined, washed with brine, dried over Na2SO4, and concentrated. The residual oil was purified by column chromatography over silica gel using hexanes/ethyl acetate (80:20) to give 6(1.92g, 91%) as a colorless oil.
Synthesis of target molecule 1
To an ice cold solution of silyl ether 7 (0.50 g, 1.61 mmol, 1 equiv.) in THF (20mL) was added TBAF (2.41 mL, 1.0 M solution in THF, 2.41 mmol, 1.5 equiv.). The solution was stirred at room temperature for 5 h and diluted with saturated NH4Cl (20 mL) and ethyl acetate (20mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (2 x 30mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure to obtain an oily residue, which was purified by chromatography over silica gel using hexanes/ethyl acetate (70:30) to afford 1 (300mg, 95% yield); [α]D20 +63.9 (c 0.12, MeOH); 1H NMR (300 MHz, CDCl3): δ 7.27- 7.32 (m, 2H), 7.19-7.24 (m, 3H), 3.77-3.87 (m, 4H), 2.83 (dd, J = 14.0, 3.7 Hz, 1H), 2.69 (dd, J = 13.9, 9.0 Hz, 1H), 1.75-1.81 (m, 2H); 13CNMR (75 MHz, CDCl3): δ 138.3, 129.3, 128.5, 126.4, 75.3, 73.4, 60.7, 38.4, 33.0; IR (KBr): νmax = 3351, 2924, 1464, 1224, 1171, 725 cm-1; MS (ESI): m/z 219 (M+Na)+; HRMS (ESI):m/z calcd for C11H16NaO3 (M+Na)+: 219.0992, found: 219.0995.
Acknowledgement
R Venkateshwarlu thankful to the Director, CSIR-IICT, Hyderabad, for their support to provide NMRs.
References
- Y Suzuki, Y Esumi, H Hyakutake, Y Kono, A Sakurai (1996) Isolation of 5-(8′Z-heptadecenyl)-resorcinol from etiolated rice seedlings as an antifungal agent. Phytochemistry 41(6): 1485-1489.
- Q Xu, AA Kulkarni, AM Sajith, D Hussein, D Brown, et al. (2018) Design, synthesis, and biological evaluation of inhibitors of the NADPH oxidase, Nox4. Bioorganic & Medicinal Chemistry 26 (5): 989-998.
- DR Motati, D Uredi, EB Watkins (2018) A general method for the metal-free, regioselective, remote C–H halogenation of 8-substituted quinolines. Chem Sci 9(7): 1782-1788.
- MD Reddy, H Kobori, T Mori, J Wu, H Kawagishi (2017) Gram-Scale, Stereoselective Synthesis and Biological Evaluation of (+)-Armillariol C. J Nat Prod 80(9): 2561-2565.
- MD Reddy, AN Blanton, EB Watkins (2017) Palladium-Catalyzed, N-(2- Aminophenyl)acetamide-Assisted Ortho-Arylation of Substituted Benzamides: Application to the Synthesis of Urolithins B, M6, and M7. J Org Chem 82(10): 5080-5095.
- JL Chen, F Zheng, Y Huang, FL Qing (2011) Synthesis of γ-Monofluorinated Goniothalamin Analogues via Regio- and Stereoselective Ring-Opening Hydrofluorination of Epoxide. J Org Chem 76(16): 6525-6533.
- SP Reddy, Y Venkateswarlu (2013) Synthesis and biological evaluation of (-)-kunstleramide and its derivatives. Tetrahedron Lett 54(35): 4617-4619.
- CR Reddy, U Dilipkumar, MD Reddy, NN Rao (2013) Org Biomol Chem 11(20): 3355-3364.
- CR Reddy, MD Reddy, U Dilipkumar (2014) Total Synthesis of a Pyrrole Lactone Alkaloid, Longanlactone. Eur J Org Chem 2014(28): 6310- 6313.
- MD Reddy, EB Watkins (2015) Palladium-Catalyzed Direct Arylation of C(sp3)–H Bonds of α-Cyano Aliphatic Amides. J Org Chem 80 (22): 11447-11459.
- C Vilanova, S Díaz Oltra, J Murga, E Falomir, M Carda, et al. (2014) Design and Synthesis of pironetin Analogue/Colchicine Hybrids and Study of their cytotoxic Activity and Mechanisms of interactions with Tubulin. J Med Chem 57(24): 10391 -10403.
- Y Liu, LH Rakotondraibe, PJ Brodie, JD Wiley, MB Cassera (2015) Antimalarial 5,6-Dihydro-α-pyrones from Cryptocarya rigidifolia: Related Bicyclic Tetrahydro-α-Pyrones Are Artifacts1. J Nat Prod 78(6): 1330-1338.
- CR Reddy, MD Reddy (2014) A Metal-Free Tandem C–C/C–O Bond Formation Approach to Diversely Functionalized Tetrasubstituted Furans. J Org Chem 79(1): 106-116.
- J Tang, Z Chen, B Sun, J Dong, J Liu, et al. (2016) Polyhydroxylated fullerenols regulate macrophage for cancer adoptive immunotherapy and greatly inhibit the tumor metastasis. Nanomedicine 12(4): 945- 954.
- JH Choi, T Suzuki, H Okumura, K Noguchi, M Kondo, et al. (2014) Mycoleptodonoides aitchisonii suppresses asthma via Th2 and Th1 cell regulation in an ovalbumin-induced asthma mouse model J Nat Prod 77(7): 1729-1733.
- N Nagesh, G Raju, R Srinivas, P Ramesh, MD Reddy, et al. (2015) A dihydroindolizino indole derivative selectively stabilizes G-quadruplex DNA and down-regulates c-MYC expression in human cancer cells. Biochim Biophys Acta 1850(1): 129-140.
- MD Reddy, FR Fronczek, EB Watkins (2016) Rh-Catalyzed, Regioselective, C–H Bond Functionalization: Access to Quinoline- Branched Amines and Dimers. Org Lett 18(21): 5620-5623.
- S Aravind, N Reddy (2015) Copper‐Exchanged Tungstophosphoric Acid: An Efficient and Reusable Heteropoly Acid for the Synthesis of 2,3,4,5‐Tetrahydro‐4‐methylidenefuran Derivatives. Helv Chim Acta 98(4): 557-560.
- R Venkateshwarlu, B Chinnababu, U Ramulu, KP Reddy, MD Reddy (2017) Synthesis and biological evaluation of (−)-kunstleramide and its derivatives. Med Chem Commun 8(2): 394-404.
- G Zhong, Y Yu (2004) Enantioselective Synthesis of Allylic Alcohols by the Sequential Aminoxylation-Olefination Reactions of Aldehydes under Ambient Conditions. Org Lett 6(10): 1637-1639.
- CR Reddy, NN Rao, MD Reddy (2012) Total Synthesis of (+)‐ Seimatopolide A. Eur J Org Chem 2012(26): 4910-4913.
- LC Dias, DP Sant Ana, YW Vieira, CCS Gonçalves, DJP Lima (2012) Synthesis of the C1-C9 fragment of the potent antitumor agent dictyostatin. J Braz Chem Soc 23(2): 344-348.
- T Katsuki, KB Sharpless (1980) The first practical method for asymmetric epoxidation. J Am Chem Soc 102(18): 5974-5976.
- SM Viti (1982) Regioselective reductions of 2,3-epoxy alcohols. Tetrahedron Lett 23(44): 4541-4544.






























