An Alternative Method for the Synthesis of S-Methylmethanethiosulfonate
Arif Ali Khan*
University School of Basic & Applied Sciences, Guru Gobind Singh Indraprastha University, India
Submission: April 23, 2018; Published: April 26, 2018
*Corresponding author: Arif Ali Khan, University School of Basic & Applied Sciences, Guru Gobind Singh Indraprastha University, Dwarka, Sector 16-C, New Delhi-110078, India, Tel: +91 98189 74597; Email: arif@ipu.edu
How to cite this article: Arif A K. An Alternative Method for the Synthesis of S-Methylmethanethiosulfonate. Organic & Medicinal Chem IJ. 2018; 6(3): 555689. DOI: 10.19080/OMCIJ.2018.06.555689
Abstract
DMSO is converted to S-Methylmethanethiosulfonate in good yields in the presence of catalytic amount of TiCl4. It provides the first example of conversion of DMSO to S-Methylmethanethiosulfonate catalysed / promoted by TiCl4. The product has been characterised by JH- and 13C-n.m.r. spectroscopy and mass spectrometry.
Keywords: DMSO; S-Methyl methanethiosulfonate; TiCl4
Abbreviations: DMSO : dimethyl sulfoxide
Introduction
DMSO and other sulfoxides are widely used in organic synthesis [1-4]. The presence of metal complexes in such reactions often results in oxidation of sulfoxides and sometime it results even in activation of Carbon-Sulfur bond of sulfoxides giving unexpected products which might lead to confusion [5]. In a similar way, a reaction of DMSO in presence of TiCl4 in 1,2-Dichloroethane resulted in the formation of S-Methylmethanethiosulfonate. S-Methylmethanethiosulfonate is a very useful sulfenylating reagent for organic synthesis as well as for biological applications [6-15]. Particularly, it is used for introduction of SCH3 groups in aromatic rings and other organic compounds including biologically active molecules. In general, thiosulfonic S-esters are stable and reactive species; however their use has been limited by the lack of easy and practical preparations. A number of methods are known for the preparation of S-Methylmethanethiosulfonate which involves harmful toxic reagents or typical lengthy work-up which distract the interest of chemists [16-24]. Herein, we report a novel and very simple method for the synthesis of the title compound.
Results and Discussion
Conversion of DMSO (1) to S-methylmethanethiosulfonate (2) is attained selectively at reflux conditions in 1,2-dichloroethane in presence of TiCl4 (Equation 1). The progress of the reactions is monitored by TLC and it takes 2-3 hrs for completion.
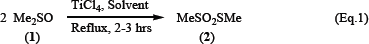
During usual work up of the reaction some brownish impurities are developed which can be easily removed by short path distillation under vacuum and as a result a neat colorless liquid (2) is obtained in 79% yield. The *H NMR of this compound shows two peaks as singlet at 2.71 and 3.32 ppm corresponding to chemically two different methyl groups indicating that the two methyl groups are low field shifted compared to DMSO which shows a single peak at 2.62 ppm. Similarly, 13C NMR spectrum of 2 shows two peaks at 18.2 and 48.8 ppm corresponding to two different methyl groups and non-decoupled 13C-nmr spectrum further shows two methyl carbon as quartet indicating the presence of three coupling protons on each carbon atoms. The fragmentation pattern of 2 obtained by EI (30 eV) shows major products corresponding to 126(M+), 111(CH3SO2S+), 79(CH3SO2+), 63(CH3SO+), and 47(CH3S+) respectively. The fragment (M= 63) is also generated in thermolysis of DMSO due to loss of methyl radical from the molecular ion [25].
Attempts will be made to explore the potential of S-Methylmethanethiosulfonate towards various organo- phosphorus compounds.
Experimental
Caution: This sulfenate should be handled carefully as it possesses a pungent smell and may cause vomiting and headache).
Synthesis of Compound (2): A mixture of DMSO (23.4 g, 0.30 mol) and TiCl4 (0.190 g, 0.009 mol) in 100 mL of freshly distilled 1,2-dichloroethane was heated at reflux for 2-3 h. After completion of the reaction (marked by a single spot of S-methylmethanethiosulfonate on TLC), the reaction mixture was cooled to room temperature and then washed with water. The 1,2-dichloroethane solution was dried (anhydrous Na2SO4 ) and evaporated on a rotary evaporator to give a light brown liquid which became dark on exposure to air. This product 2 was distilled by short path distillation under vacuum (13.60 g, 70.6%; b.pt. 80-82 °C/ 0.05 mmHg) as a neat colorless liquid. Calcd. Anal.: C, 19.03; H, 4.79; Found: C, 18.99; H, 4.78. ^ NMR(300 MHz, CDCl3) 2.71(s,3H), 3.32(s,3H); 13C NMR(75 MHz, CDCl3)18.2, 48.8; MS (EI, 30 eV)m/z= 126 (62%, M+), 111(10%, CH3SO2S+), 79(60%,CH3S02+) 63 (61%, CH3SO+), 47(100%,CH3S+).
(P.S.: 1,2-Dichloroethane was used as freshly distilled. DMSO and TiCl4 were used as received).
Acknowledgement
Author is thankful to CSIR-New Delhi for financial assistance.
References
- GH Posner (1988) The Chemistry of Sulfones and Sulfoxides. Wiley, New York, USA, 3: 55.
- A Kalir, HH Kalir (1993) In the Chemistry of Sulfur-Containing Functional Groups, supplements. Patai S, Z Rappoport, Wiley, New York, USA, pp. 957.
- MC Carreno (1995) Applications of Sulfoxides to Asymmetric Synthesis of Biologically Active Compounds Chem Rev 95(6): 1717-1760.
- I Fernandez, N Khiar (2003) Recent Developments in the Synthesis and Utilization of Chiral Sulfoxides Chem Rev 103(9): 3651-3705.
- SR Dubbaka, P Vogel (2005) Palladium-Catalyzed Suzuki-Miyaura Cross- Couplings of Sulfonyl Chlorides and Boronic Acids. Angew Chem 117: 7848-7859.
- Zefirov NS, Zyk NV, Beloglazkina EK, Kutateladze AG (1993) Sulfur Rep 14: 223
- Trost BM (1978) alpha-Sulfenylated carbonyl compounds in organic synthesis. Chem Rev 78(4): 363-382.
- Palumbo G, Ferreri C, D Ambrosio C, Caputo R (1984) Phosphorus Sulfur 19: 235.
- Bosscher JK, Kraak EWA, Klooaterziel HJ (1971) Chem SOC D 21: 1365.
- Dunbar JE, Harris RF, Mc Carthy JR (1972) Chem Abstr 82: 72796.
- Smith DJ, Maggio ET, Kenyon GL (1975) Biochemistry 14: 766-771.
- Applegate HE, Cimarusti CM, Dolfini JE, Funke PT, Koster WH, Puar MS, et al. (1979) Synthesis of 2-, 4-, and 7-methylthio-substituted cephalosporins. Org Chem 44(4): 811-818.
- Slusarchyk WA, Applegate HE, Funke P, Koster W, Puar MS (1973) Org Chem 38: 943-950.
- Ito Yoshiaki, Nakamura Yasushi, Nakamura Yoshiyuki (1997) Suppression of aflatoxin B1- or methyl methanesulfonate-induced chromosome aberrations in rat bone marrow cells after treatment with S-methyl methanethiosulfonate. Mutation Research 393(3): 307-316.
- Nakamura Yasushi K, Kawai Kazuaki, Furukawa Hideyuki, Matsuo Tomoaki, Shimoi Kayoko (1997) Mutation Research 385(1): 41-46.
- Freeman F, Bartosik LG, van Bui N, Keindl MC, Nelson EL (1988) Phosphorus Sulfur 35: 375.
- Freeman F (1984) Chem Rev 84: 117.
- Freeman F, Keindl MC (1983) Synthesis pp. 913.
- Palumbo G, Caputo R (1981) Synthesis pp. 888.
- Tsuchiya T, Iriyama S, Umezawa S (1963) Jpn Chem Soc Bull 37: 286.
- Meier H, Menzel I (1972) Synthesis pp. 267.
- Chemla F (1998) Synlett pp. 894.
- Chemla F, Karoyan P (2004) Organic Syntheses Coll 546(78): 99.
- Laszlo P, Mathy AJ (1984) Org Chem 49: 2281.
- Griffiths IW, Howe I, March RE, Beynon JH (1983) Int J Mass Spectrom Ion Processes 54: 323.






























