Alkylation of N,N-dimethylethanolamine with Benzhydryl Halogenide in PTC Condition
GH Torosyan*
National polytechnic university of Armenia, Yerevan
Submission: March 27, 2018; Published: April 06, 2018
*Corresponding author: GH Torosyan, National polytechnic university of Armenia, Armenia, Yerevan Gagik Torosyan, Doctor of Chemical sciences, Tel: 00374 93 998830; Email: gagiktorosyan@seua.am
How to cite this article: GH Torosyan. Alkylation of N,N-dimethylethanolamine with Benzhydryl Halogenide in PTC Condition.Organic & Medicinal Chem IJ. 2018; 6(2): 555684. DOI: 10.19080/OMCIJ.2018.05.555684
Abstract
The direct O- alkylation of dimethylethanolamine (DMEA) by benzhydryl chloride (BhHlg) as electrophilic reagent was performed in Phase Transfer Catalysis system (PTC). It was presented the possibilities for best yield of O-alkylated product - Diphenhydramine in PTC process. The hydrochloride of obtained amino-ether is a widely known drug - dimedrol.
Keywords: Dimethylethanolamine; Alkylation; Benzhydryl Chloride (chloro or bromodiphenylmethane); Phase transfer catalysis (PTC); TBAB; Diphenhydramine; Dimedrol.
Abbreviations: DMEA: Dimethylethanolamine; BhHlg: Benzhydryl Chloride; PTC: Phase Transfer Catalysis
Introduction
Diphenhydramine is an important compound which has more application in pharmaceutical industry. It is use as antihistamine for treat allergies, for insomnia, for tremor in nausea, also as injection into a vein and a muscle and others [1]. Here is presented an O-alkylation of DMEA by benzhydryl halogenide (chloro- r bromodiphenylmethane).
Results and Discussion
It was previously established that the alkylation of MEA with alkyl halides leads to the formation of mono- N and di-N, N alkylation products, the yield of which depends on the ratio of monoethanolamine and alkyl halide [2]. The chlorhydrate of diphenhydramine - dimedrol is one of the main representatives of a group of antihistamines blocking H1-receptors.
Alkylation of dimethylethanolamine with benzhydryl chloride was carried out in a two-phase “liquid-liquid” and "solid phase-liquid” PTC system. It turned out that in the system “liquid-liquid” the yield of the O-alkylation product are low and along with the amine symmetrical ether of benzhydrol is obtained. The latter is obtained by hydrolysis of the starting alkyl halide with further alkylation with the same halide. Previously we had synthesized dibenzyl ether under the same conditions from benzyl chloride [3].
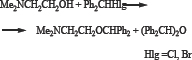
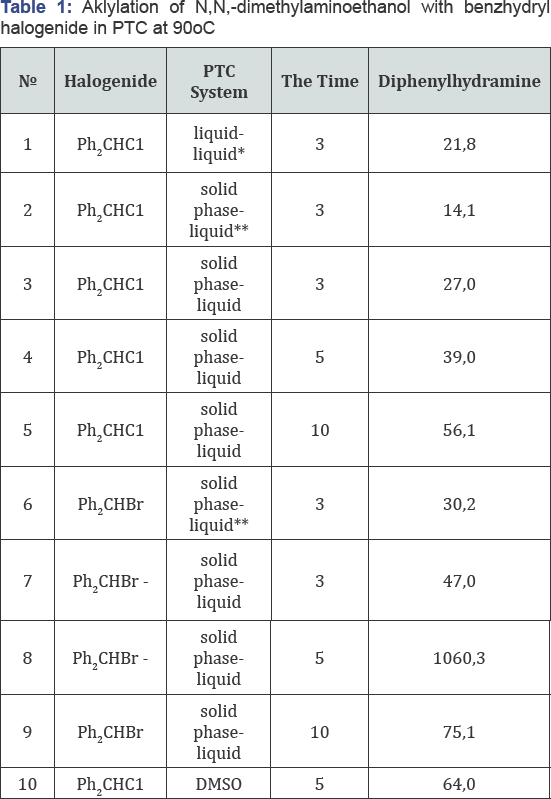
It follows from the table that the yield of diphenhydramine also depends on the nature of the alkyl halide (Table 1). Symmetrical ether is not formed in the “solid phase-liquid” PTC system.
Experimental Part
A mixture of DMEA, an aqueous solution of potassium hydroxide (or powdered potassium hydroxide) and quaternary ammonium salt - Quat (during the reaction in the "liquid-liquid” system 50% aqueous solution of catamine AB, in the "solid phase-liquid” system tetrabutylammonium bromide is used) was stirred at 90-95°C in for 15 minutes. After it had been added benzhydryl halogenide. The molar ratio of DMEA: BhHlg: KOH: Quat = 1: 1: 1-1.5: 1. The heating and stirring continued 3-10 (The reaction duration indicated in the table) (Table 1). Then the flask was cooled to 10-15°C, extracted three times with diethyl ether, the ether extract was dried over sodium sulfide. After removal of the ether, the reaction products were isolated by distillation.
When the reaction was carried out in DMSO, after cooling the reaction mixture was washed with water (20-30 ml). The organic mass was treated in the same manner
Received Diphenhydramine
b.p. 155-158oC/ 3mm, Nd20 1.5 5 5 2, m.p. hydrochloride 165- 167°C, NMR spectrum: 2.08s (6H, 2CH3); 2,41t (2H, NCH2); 3,40, (2H, CH2O); 5,02 (1H) 7,18 l (10H, C6H5) [4]. IR spectrum ν: 1500, 1600, 3030, 3080 (C6H5), 1120 (C-O-C), UV spectrum λ: 254, 258, 265 [4]. In the system, obtained benzhydrol b.p. 162-165oC/10mm, m.p.65-66, obtained symmetrical benzhydryl ether m.p. 107-108oC [4].
References
- (2016) Diphenhydramine. International Drug Price Indicator Guide.
- Torosyan GH (2018) The selective N-alkylation of monoethanlamine in PTC condition. MOJ Bioorganic & Inorganic Chemistry 2(1): 00049.
- Babayan AT, Torosyan GH (1986) The Stage of PTC development. The Journal of Mendeleev Society Revue 12: 126-135.
- GH Torosyan (1987) Reaction of inter- and internal alkylation for compounds with carbonyl and hydroxyl groups. Thesis for chemical doctor degree, Yerevan, Armenia p. 358.






























