Kinetic Studies of Charge Transfer Formation of Erythromycin and 2, 3 Dichloro-5, 6-Dicyano -1,4- Benzoquinone
Nwanisobi Gloria1* and Ukoha Pius2
1Department of Chemical Engineering, Madonna University Nigeria, Nigeria
2Department of Pure and Industrial Chemistry, University of Nigeria, Nigeria
Submission: March 20, 2018 Published: April 04, 2018
*Corresponding author: Nwanisobi Gloria, Department of Chemical Engineering, Madonna University Nigeria, Akpugo campus, Nigeria; Tel: +2348052240626; Email: glochinwa4real@yahoo.com
How to cite this article: Nwanisobi G Ukoha P. Kinetic Studies of Charge Transfer Formation of Erythromycin and 2,3 Dichloro-5,6-Dicyano -1,4- Benzoquinone. Organic & Medicinal Chem IJ. 2018; 6(2): 55581. DOI: 10.19080/OMCIJ.2018.06.555681
Abstract
The kinetics of erythromycin with 2, 3 dichloro-5,6-dicyano -1,4- benzoquinone (DDQ) is described for the determination of erythromycin. It was carried in aqueous HC1O4 (2M) medium at ionic strength of 0.01moldm-3, Kinetics of the reactions infer that the rate of formation of the charge transfer complex did not vary significantly with increase in concentration of erythromycin indicating likely zeroth order dependence of the rate with respect to concentration of the drug. However, the linearity of the pseudo-first order plot points to first order dependence of rate on [DDQ].The overall rate equation for the reactions can be given as
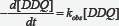
Keywords: Kinetic studies; Erythromycin; Charge transfer; DDQ
Introduction
The kinetic methods of analysis are highly sensitive, selective, simple, accurate and less expensive [1]. Erythromycin, 3R, 45 55, 6R, 7R, 9R, 11R, 12R, 13R, 14R - 4-[(2,6-didiocy -3-c-methy 1-3-0-methy 1 -a-l-ribo -hexopy- ransyi) oxy]- 14 ethy 1-7, 12,13-trihydroxy- 3, 5,7,9,11,13-hexamethy 1-6-[(3,4,6-trideoxy -3-dimethy kmiro - P-D-xylo-hexopy ransoyl) -oxy] oxa - cydofetradecare -2, 10-dione is a macrolide antibiotic used for the treatment of urinary tract infection. It targets at the ribosome and inhibits the protein synthesis of gram positive bacteria such as mycoplasma and Chlamydia [2]. In recent years, several kinetic catalytic techniques have been reported for the detection of biomolecules [3,4]. Literature revealed different techniques for the analysis of erythromycin as follows: spectrofluorimetry [5]. Capillary electrophoresis [6], HPLC [7], microbiological method [8] spectrophotometry [9,10]. Therefore, the aim of this research is to determine a kinetic method based on formation of charge transfer complex between erythromycin and DDQ that is simple, fast, economical and less laborious.
Materials and Methods
Equipment
All kinetic measurements were carried out using a UV- 1800 Shidmazu and 752w UV - Vis grating with a silica glass cell of l cm thickness. All chemicals were of analytical grade and were used as such. Erythromycin powder was supplied by AC pharmaceutical limited, Enugu, Nigeria. The commercial erythromycin tablet (500mg per tablet) was purchased from the local market (Syncom formulations limited, India) 2,3 dichloro - 5, 6- dicyano-1,4-benzoquinone (98% purity) was supplied by sigma Aldrich, Germany.
Kinetic Measurements
Kinetics of the reactions of DDQ with erythromycin was followed spectrophotometrically under pseudo-first order condition with one of the reactants (donor) in at least 10 fold in excess over the reactant (acceptor) at 290C. The pseudofirst order rate constants were determined by using various concentrations of erythromycin (0.001 M to 0.0035 M) with fixed concentration of acceptor (2.5 x10-4 M). Effects of ionic strength, pH and hydrogen ion on the rates of reaction were also determined.
The pseudo-first order rates constant were obtained from a plot of log versus time following equation 1.
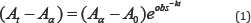
Where Aα and A 0are the final and initial absorbencies respectively, is absorbance at time t and is pseudo-first order rate constant.
Effect of Hydrogen Ion Concentration on the Rate of Erythromycin and DDQ Reaction
Within the hydrogen ion concentration range 1.0 x10- 2 to 1.0mol dm-3, kinetic runs were carried out keeping the concentrations of the drug and reagent constant at I =1.02mol dm-3 and T= 30.0± 0.20C.
Effect of Ionic Strength on the Rate of Erythromycin- DDQ Reaction
Within the range of ionic strength of the media 0.001- 0.03mol dm-3, the variation of rate of reaction with ionic strength was investigated for the reaction of erythromycin and DDQ, At T = 280C and keeping the concentrations of drug and reagent constant, the ionic strength was varied and the rate of plot isreseprented in Figure 1. reaction monitored.
Results and Discussion
Reaction of Erythromycin with DDQ
The wavelength of maximum absorption spectrum was found to be 464nm with a stoiochiometric ratio of 1:1 of erythromycin - DDQ complex [11].
Kinetic Studies
The rate of the charge transfer reaction of erythromycin and DDQ was followed spectrophotometrically under pseudo - first order conditions by monitoring the rate of consumption of DDQ at 464nm. Erythromycin was at least 10 fold in excess of DDQ and the reaction was carried out at pH 8.Kinetic decays were exponential and pseudo first rate constants were determined from the plots of log versus time according to equation 1. The plots were linear for more than 90 % extent of reaction. Typical plot is represented in Figure 1.
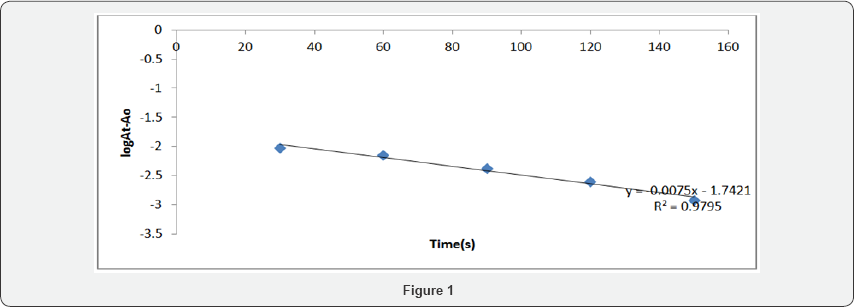
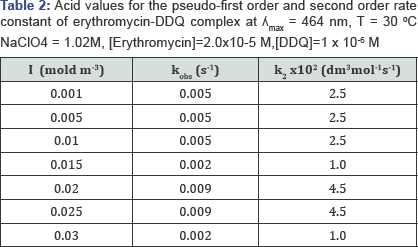
NaClO4 = 1.02M, [Erythromycin] = 2.0x10-5 M, [DDQ] = 1 x 10-6 M
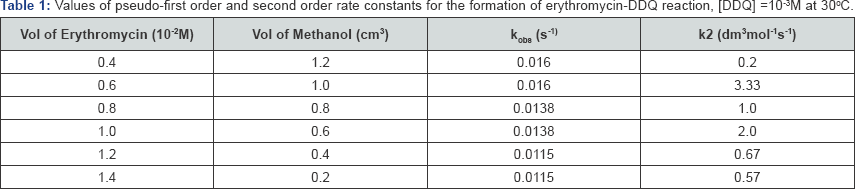
The second order rate constants were determined by dividing Kobs with [erythromycin]. The values of Kobs and k2 are represented in Table 1. The result from table 1 shows that as concentration of erythromycin increased from 0.001M to 0.0035M, the Kobs values did not vary significantly indicating a zero order dependence on reaction rate with respect to the concentration of erythromycin. However, the linearity of the pseudo - first order plot points to first - order dependence of rate with respect to [DDQ].
Effect of Hydrogen Ion Concentration on Erythromycin - DDQ Complex
The H+ concentration was varied from 0.08M to 1M while keeping other variables constant. Table 2 shows that Kobs increase from 0.08M to 1M. This infers that within this acid concentration range, protonation of the reactants played significant role in the charge transfer reaction. The Second order rate constants were determined by dividing Kobs with[erythromycin]. Table 2 is a display of pseudo-first order and second order rate constants.
Effect of Ionic Strength on Erythromycin - DDQ Complex
In addition, ionic strength medium was varied from 0.001M to 0.03M. Table 3 shows that Kobs did not vary significantly at 0.001M-0.01M but increased from 0.015M to 0.025M of the varied ionic strength which indicates primary salt effect and likely involvement of charged partners at the rate determining step.

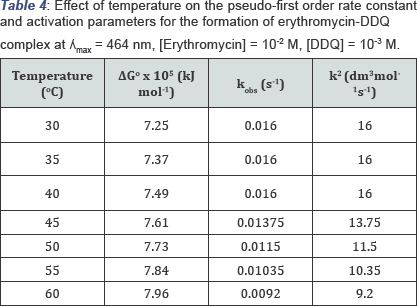
Effect of Temperature on Erythromycin - DDQ Complex
Effect of temperature was studied between 303k- 333k. Result in (Table 4) shows a drop in Kobs from 313k to 333k, this shows that as the temperature increases, the rate of reaction decreased. Also, Table 4 reveals a negative entropy, negative enthalpy change and positive Gibb's free energy change which means that the reaction does not occur spontaneously
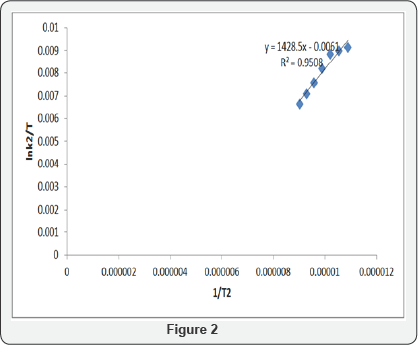
Least square fit of log K_obs/T 1/Tvs based on the Eyring - Polanyi (equation 2) is represented in figure 2.
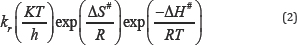
Where, kr = rate constant, k = Boltzmann's constant, h = Planck's constant, ΔS# = entropy of activation ΔH# = enthalpy of activation, T = absolute temperature, R = gas constant.
The rate determining steps of erythromycin-DDQ complex are as follows:
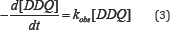
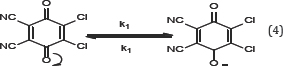
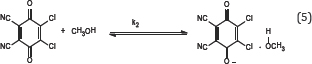
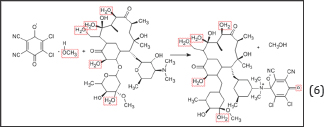
ΔS# (kJk-1/mol) = -2391; ΔH# (kJ mol-1) = -11
Conclusion
The proposed kinetic method infer that the rate of formation of charge transfer complexes did not vary significantly with increase in concentration of erythromycin indicating a likely zeroth order dependence of the rate with respect to the concentration of erythromycin. However, the linearity of the pseudo - first order plot points to first order dependence of rate on [DDQ].
References
- Mohammed l, Maqsood A, Syed M, Zaheer K (2007) Kinetics and mechanism of paracetamol oxidation by chromium (VI) in absence and presence of manganese (II) and sodiumdodecyl sulphate Research letters in physical chemistry.
- Onura S Macrolide Antibiotics Chemistry, Biology and Practice. Academic Press Orlando, USA, p. 26.
- Nandibewoor ST, Morah VA (1995) Chromium (III) Catalysed Oxidation of Antimony (III) by Alkaline hexacyanoferrate (III) and Analysis of chromium (III) in microamounts by kinetic method. Journal of the chemical society, Dalton 3: 483-488.
- Imdadullar TF, Karmamaru (1994) Catalytic Effect of Rhodium (III) on the Chemiluminescence of Luminal in Reverse Miscelles and its Analytical Application. Analytica Chimica Acta 292(1-2): 151-157.
- Pakinaz YK (2002) Spectrophotometric Analysis of Certain Macrolide Antibiotics in bulk and Pharmaceutical Formulation. Journal of Pharmaceutical and Biomedical Analysis 27(6): 923-932.
- Nawal R, Hassan F, Pakiraz K, Noha N, Attia A (2006) Validated Spectrophotometric Assay for the Determination of Certain Macrolide Antibiotics in Pharmaceutical Formations and Spiked Biological Fluidss, J AOAC Int 89(5): 1276-1287.
- Maria H, Britt E (1995) Liquid Chromatographic Determination of the Macrolide Antibiotics Toxithromycin and Clarithromycin in Plasma by Automated Solid-Phase Extraction and Electrochemical Detection. J Chromatogr A 692 (1-2): 161-166.
- Bernabeu JA, Camacho MA, Gilalegre ME, Ruz V, Towes S (1999) Microbiology Bioassay of Erythromycin Thiocyanate: Optimization and Validation. J Pharm biomed Anal 21(2): 347-353.
- Carlos E, Paula R, Vanessa GK, Almeida R, Cassella J (2010) Novel Spectrophotometric Method for the Determination of Azithromycin in Pharmaceutical Formulations based on its Charge Transfer Reaction with Quinalization. Journal of Brazillian Chemical Society 21: 16641671.
- Safwan A, Roula B (2012) Novel Spectrophotometric Method for Determination of some Macrolide Antibiotics in Pharmaceutical Formulations using 1, 2- naphthoquinone-4- sulphonatae. Spectrochim Acta Part A 99: 74-80.
- Ukoha O Pius, Nwanisobi C Gloria (2016) Spectrophotometric Determination of Erythromycin using Charge Transfer Complexation. Der Pharma Chemica 8(2): 59-66.
- Petr P, Tomas O, Frantisek S (2016) Introduction of Novel Kinetic approach to Calculation of Activation Energy and its Application to the Sinter-Crystallization of Strontian Feldspar. Ceramics International 42(15): 16969-16980.






























