Synthesis of Oxindoles, Spirooxindoles, and Isatins with Prominent Bioactive Probes: Review
Krishna Sarma Pathy* and Sriraman Chakaravathy
Department of Chemistry, IPL research center Lucknow, sriramanchakravarthy, Consultant API, vadodara, india
Submission: March 16, 2018; Published: March 22, 2018
*Corresponding author: Krishna Sarma Pathy, Department of Chemistry, IPL research center Lucknow, sriramanchakravarthy, Consultant API, vadodara, india, India; Email: drkrishnasarmapathy@yahoo.in
How to cite this article: Krishna Sarma Pathy, Sriraman Chakaravathy. Synthesis of Sildenafil Citrate Validated HPLC Method and Its Pharmaceutical Dosage Forms. Organic & Medicinal Chem IJ. 2018; 5(5): 555674. DOI: 10.19080/OMCIJ.2018.05.555674
Abstract
The present study describes analytical method of 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4- ethoxyphenyl]sulphonyl]-4methypiperazine citrate (sildenafil Citrate). The following specification particularly describes and ascertains the nature of this invention, and the manner in which it is to be performed. Sildenafil is an oral drug used primarily to treat male sexual function problems (impotence or erectile dysfunction) since becoming available in 1998. It is a potent and selective inhibitor of cGMP specific Phosphodiesterase Type 5 (PDE5) in the corpus cavernosum, where PDE5 is responsible for degradation of cGMP. Sildenafil has a peripheral site of action on erections. This substance has no direct relaxant effect on isolated human corpus cavernosum but potently enhances the relaxant effect of nitric oxide on this tissue. However, there is no analytical method for determination of this active compound in pharmaceutical preparations in the current European and US Pharmacopoeia. The aim of this study was to develop and validate HPLC method for sildenafil analysis in pharmaceutical dosage forms.
Keywords: Chlorosulphonyl Intermediate; N-Methylpiperazine; Sildenafil; IR; HPLC Validation
Abbreviations: PDE5: Phosphodiesterase Type 5; GMP: Guanosine Monophosphate; LOD: Limit of Detection; LOQ: Limit of Quantification; cGMP: Cyclicguanosine Monophosphate
Introduction
Sildenafil citrate is a selective inhibitor of cyclic Guanosine Monophosphate (GMP) or Specific phosphodiesterase type 5 (PDE 5), commercially developed by Pfizer, Inc._as Viagra®. Sildenafil citrate is designated chemically as 1-[[3-(6,7-dihydro- 1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4- ethoxyphenyl]sulphonyl]-4-methypiperazine citrate (Figure 1) [1]. The compound has the following structure:
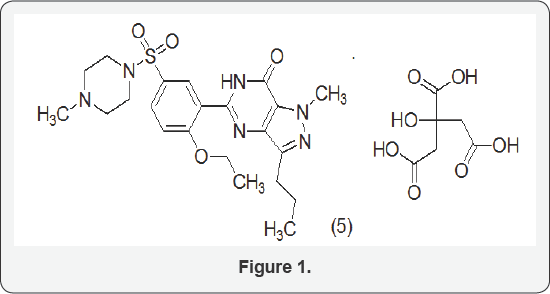
The manufacture of Sildenafil citrate has been desribed in various patents and to cite a few references, EP 1002798, EP 1779852, EP0916675,US6066735,US6204383, US2010048897, WO0119827, WO122918, and WO2004072079.
With respect to polymorphic forms of sildenafil citrate, while there are no patents reported, but in a publication describe three polymorphic forms. The process for the preparation of sildenafil citrate of polymorphic form I as designated (Figure 2).
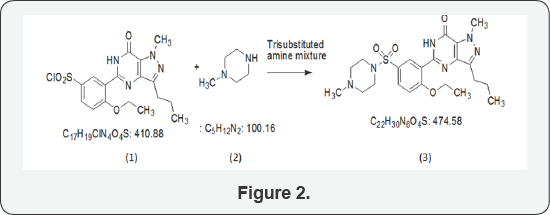
The process is from the penultimate intermediate namely 5-(5-chlorosulphonyl-2-ethoxy phenyl) -1-methyl-3-N-propyl- 1,6-dihydro-7H-pyrasolo-(4,3-d)pyrimidin-7-one, which is herein will be referred to as chlorosulphonyl intermediate (21). This intermediate is condensed with N-methylpiperazine (2) in a solvent preferably of chlorinated hydrocarbon in presence of a trisubstituted amine or in presence of mixture of such amines (Figure 3) [2].
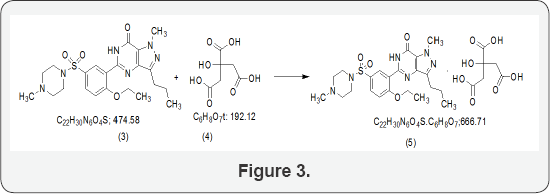
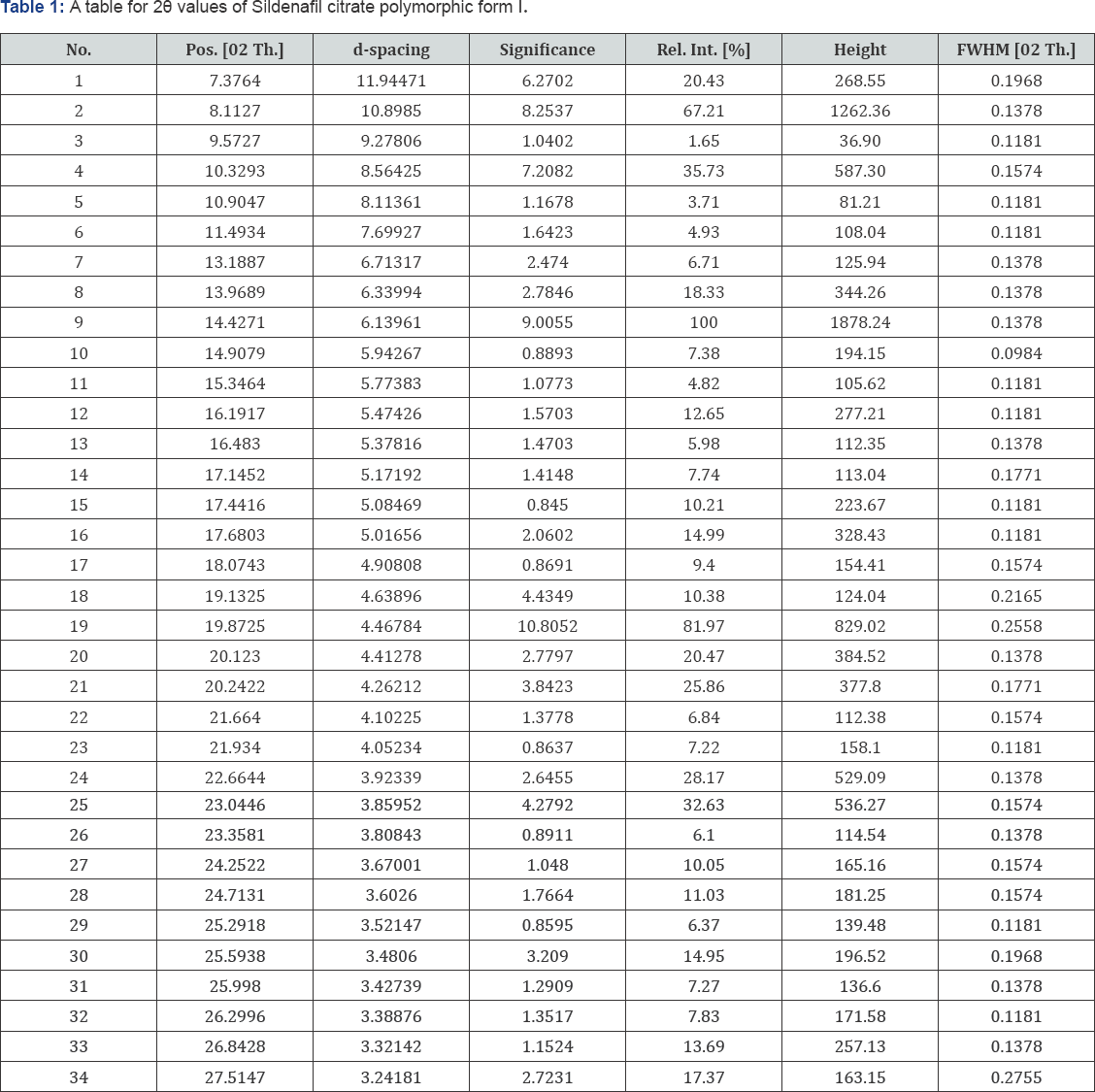
The resulting product of condensation namely sildenafil base is reacted with citric acid in an aqueous medium to give sildenafil citrate (3). The crystallization conditions are well established to give crystalline form I [3]. The powder X-ray diffraction pattern of the sildenafil citrate polymorphic form I as given in Figure 1 and the 2θ values are given in Table 1.
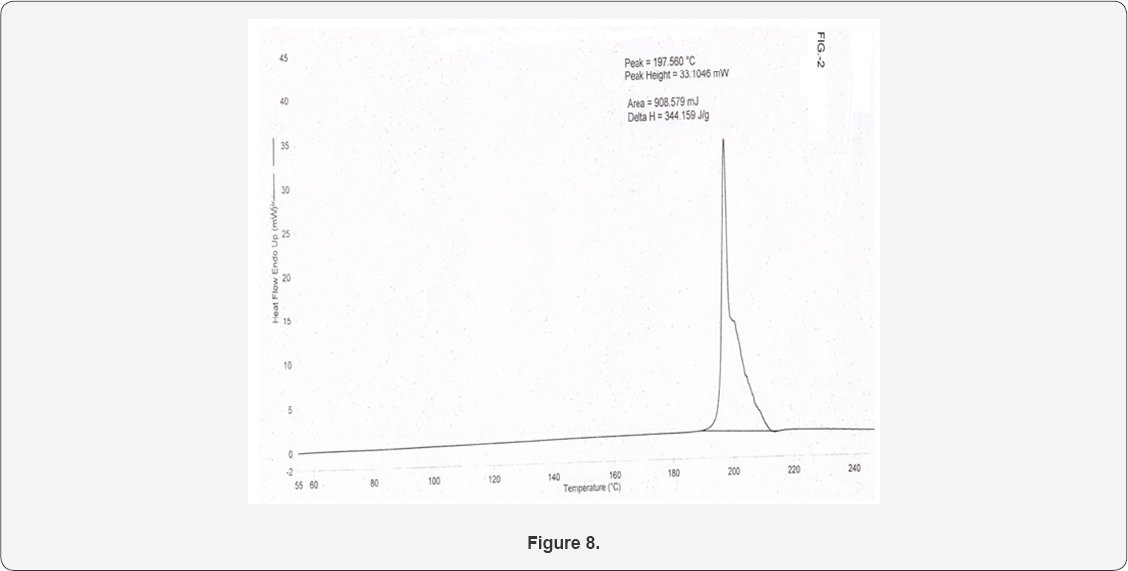
The Differential scanning calorimeter graph of the Sildenafil citrate polymorph I under specific conditions shows the melting point around 197.56_°C. Figure 3 depicts a comparison of DSC scanned at 5_°C/min over a temperature range of 30_°C to 350_°C for sildenafil citrate polymorphic form 1 [4].
Materials and Methods
HPLC analysis was performed using a Schimadzu LC-2010 chromatographic system (Schimadzu, Kyoto, Japan) consisting of a LC-20AT Prominence liquid chromatography pump with DGU- 20A5 Prominence degasser, a SPD-M20A Prominence Diode Array Detector, RF 10AXI fluorescence detector and a SIL-20 AC Prominence auto sampler. Data analyses were done using Class VP 7.3 Software [5,6]. The elution was carried out on a column Hypersil BDS-C18 (125 x 4 mm i.d., 5 mm), mobile phase consisted of phosphate buffer (20 mM, pH 2.8)-acetonitrile (71:29, V/V), flow rate 1.5 mL min-1, at controlled temperature (25_oC) and auto sampler temperature at 4_oC. Detection of sildenafil was carried out at 285 nm. Commercially available, film-coated tablets, containing 50 mg sildenafil as sildenafil citrate, were used in this study [7,8].
Results and Discussion
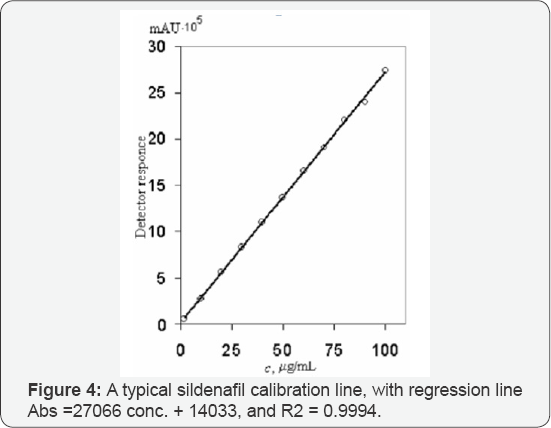
The method was fully validated according to the ICH (International Conference on Harmonization) guidelines by determination of linearity, precision, accuracy, limit of detection and limit of quantification. Linearity of the method was tested in the range of: 2 - 100 mg mL-1 sildenafil [9]. Experimental data showed high level of linearity which was proved with the value for the correlation coefficient (R2 =0.9994).
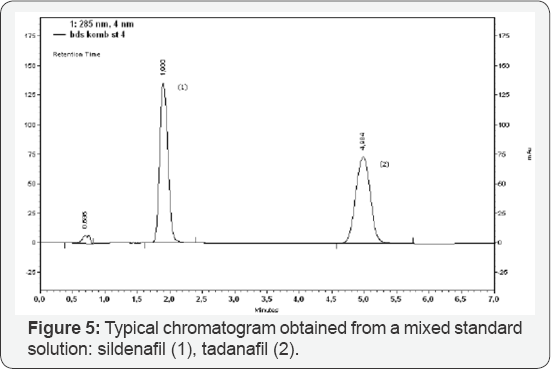
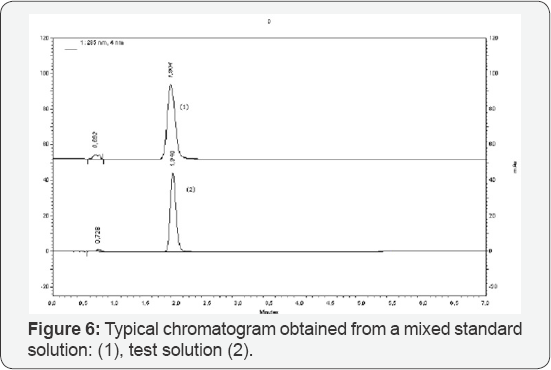
Limit of Detection (LOD) and Quantification (LOQ) of the method were tested in the range of: 20 - 200 ng μL-1 sildenafil. The results were: 0.23 ng and 0.68 ng for LOD and LOQ, respectively (9.2 ng μL-1 and 27.2 ng mL-1 for LOD and LOQ, respectively, obtained with 25 μL injected) [10,11]. Selectivity of the method was proved with the chromatographic peak resolution obtained between sildenafil and tadanafil (Rs = 10, 5) (Figures 4-5) and the characteristic UV-spectrum. Mean recovery for sildenafil was between 99,74_% and 100,88_% indicating that the developed method was accurate for determination of sildenafil in pharmaceutical formulation [12,13]. The proposed method was successfully applied for determination of sildenafil in film-coated tablets, containing 50 mg sildenafil as sildenafil citrate (Figure 5) [14-16]. The details are further illustrated in the following examples.
Example 1: General Preparation of Sildenafil Base
In a 10 Litre 3-necked flask, equipped with stirrer, thermometer and reflux condenser, methylenedichloride (6.6Litre) was charged and 4-ethoxy-3-(1-methyl-7-oxo-3- propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)benzene- 1-sulfonylchloride (823_gm; 2x103mmoles) was added at 25-30_°C. After the dissolution N-methyl piperazine(240_gm; 39x103mmoles)was added at 25-30_°C in 15-20 min.The reaction mixture was stirred properly and diisopropyl ethyl amine (262.5_gm; 2.03x103 mmoles) was added, the resultant mixture was maintained at 20_°C to 30_°C for 2.5 h. Methylene dichloride was distilled under atmospheric pressure760mm/ Hg. Charge deionized water (1.64 Litre) in residue and stir to form slurry, which was filtered and product was washed with deionized water (0.82 Litre) to give a wet sildenafil base. The wet product was dried under vacuum of about 10mmHg at 65_oC for 10 hrs to give sildenafil base 827_gm (HPLC purity-99.5_% and molar yield of 87_%).
Example 2:
In a 10 Litre 3-necked flask equipped with stirrer, thermometer and reflux condenser, methylene dichloride (6.6Litre) was charged and 4-ethoxy-3-(1-methyl-7-oxo-3- propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)benzene- 1-sulfonylchloride (823_gm; 2x103mmoles) was added at 25- 30_°C . After the dissolution add N-methyl piperazine (240_gm; 2.39x103mmoles) at 25-30_°C in 15-20 mins. The reaction mixture was stirred properly and a mixture of diisopropyl ethyl amine(335_gm; 2.59x103mmoles) and triethyl amine (262.5_ gm; 2.59x103mmoles) was added. The resultant mixture was maintained at ambient temperature for 2.5_h. Methylene dichloride was distilled under atmosphericpressure760mm/ Hg. Charge deionized water (1.64 Litre) in residue and stir to form slurry, which was filtered and product was washed with deionized water (0.82 Litre) to give a wet Sildenafil base. The wet product was dried under vacuum of 10mmHg at 65_oC for 10 hrs to give sildenafil base 779 _gm (HPLC purity-99.5_% and molar yield of 82_%).
Example 3:
In a 10 Litre 3-necked flask equipped with stirrer, thermometer and reflux condenser, methylene dichloride (6.6Litre) was charged and 4-ethoxy-3-(1-methyl-7-oxo-3- propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)benzene- 1-sulfonylchloride (823_gm; 2x103mmoles) was added 25- 30_°C. After the dissolution add N-methyl piperazine (240_gm; 2.39x103mmoles) at 25-30_°C in 15-20 mins. The reaction mixture was stirred properly and a mixture of diisopropyl ethyl amine (52.6_gm; 0.406x103mmoles) and triethylamine (164.6_ gm; 1.626x103mmoles) was added.The resultant mixture was maintained at 25_°C to 30_°C temperature for 2.5_h. Methylene dichloride was distilled under atmospheric pressure760mm/ Hg. Charge deionized water (1.64 Litre) in residue and stir to form slurry, which was filtered and product was washed with water (0.82 Litre) to give a wet sildenafil base. The wet product was dried under vacuum of 10mmHg at 65_oC for 10 hrs to give sildenafil base 872_gm (HPLC purity-99.8_% and molar yield of 91.7_%).
Example 4: Synthesis of Sildenafil Citrate (Form I)
In a 50-Litre glass assembly, deionised water (21 Litre) was charged and sildenafil base (840_gm; 1.769x103mmoles) was added to it at 25-30_0C. The reaction mixture was heated to 60- 65_°C for 1 h. Citric acid (370_gm; 1.76x103mmoles) was added to the pre heated reaction mixture. The resultant mixture was further heated up and maintained at 80-85_°C, for 1h and then charcoal treatment given at same temperature. Filter the reaction mass. Filtrate was allowed to cooled to 10-15_°C, resultant product obtained was filtered and washed with deionised water (0.84 Litre). The product was dried in vacuum (about 10 mm Hg) at 75_oC as a polymorphic form I of sildenafil citrate salt 1.0_ kg. (HPLC purity-99.9_% and molar yield of 85_%).
Example 5: Synthesis of Sildenafil Citrate (Form I)
In a 500 Litre SS reactor, 4-ethoxy-3-(1-methyl-7-oxo-3- propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)benzene- 1-sulfonyl chloride (30_kg) was mixed with methylene dichloride (240Litre) at 25_°C to 30_°C temperature, followed by addition of N-methyl piperazine (8.1kg) at 25-30_°C in 45-60 mins. After the addition the reaction mixture was stirred properly and a mixture of diisopropyl ethyl amine (2.0_kg) and triethyl amine (6.0_kg) was added, the resultant mixture was maintained at 25_°C to 30_°C temperature for 3-4_h. Methylene dichloride was distilled under atmospheric pressure760mm/Hg. Charge deionized water (60 Litre) in residue and stir properly to form slurry, which was filtered and the product was washed with deionised water (30 Litre) to give a wet sildenafil base. The wet product was dried under vacuum of 10mmHg to give sildenafil base 33.0_ kg (HPLC purity-99.8_% and molar yield of 95_%).
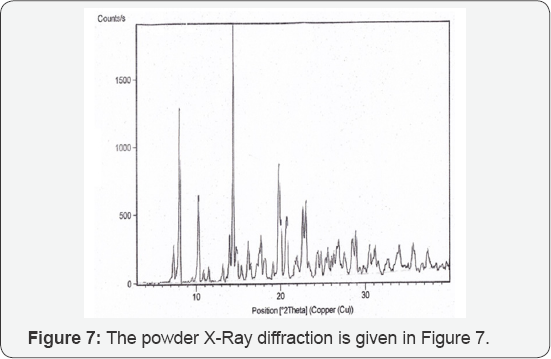
In a 1200 Litre SS reactor, sildenafil base (30kg) was mixed with water (750Litre.) at 25-30°C and the reaction mixture heated to 60-65_°C. Citric acid (13.2kg) was added to the pre heated reaction mixture and the resultant mixture was further heated to 80-85_°C for 1h.The reaction mixture was treated with carbon charcoal and then filtered. The filtrate obtained was cooled to 10-15°C, resultant product obtained was filtered and washed with deionised water. The product was dried in vacuum (10mm Hg) at 75_oC as a polymorphic form I of sildenafil citrate salt 35.5-36_kg. (HPLC purity-99.9_% and molar yield of 85_%) (Table 1) (Figures 7,8).
Conclusion
The present process, which describes the manufacturing process of sildenafil citrate, which e is a selective inhibitor of Cyclicguanosine Monophosphate (cGMP) specific Phosphodiesterase Type 5 (PDE 5), has the advantage of scaling up to the industrial level of production. The results of the validation demonstrated that the proposed analytical procedure is accurate, precise and reproducible for sildenafil analysis in pharmaceutical dosage forms. Furthermore, this procedure is relatively inexpensive and simple and is particularly suitable for routine analyses when tandem mass spectrometric detection is not available.
Additionally, it is important to mention that decreased consumption of organic solvent considerably reduces the laboratory expenses. The process uses safe reagents in the process which makes it better for industrial scale operations. The yields in the process are high which makes it a cost effective process. Residual solvents play a very important role in the impurity profile of APIs as per the ICH Guidelines ICH Q3C_(R4). In this process by carrying out the final step of condensation of Sildenafil base and citric acid in the aqueous medium followed by water crystallization.
References
- Breslow R (1996) The Greening of Chemistry. Chem & Eng News pp. 72.
- E Ernst (2002) Adulteration of Chinese herbal medicines with synthetic drugs, a systematic review. J Intern Med 252(2): 107-113.
- Anastas PT, Williamson TC (1998) Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes. Oxford University Press: Oxford, UK, Europe.
- Anastas P, Warner J (1998) Green Chemistry: Theory and Practice. Oxford University Press: Oxford, UK, Europe.
- Sheldon RA (1992) Organic synthesis; past, present and future. Chem Ind 23: 903-906.
- Sheldon RA (1994) Consider the environmental quotient. Chem Tech 24: 38-47.
- Jimenez-Gonzalez C, Curzons AD, Constable DJC, Cunningham VL (2004) Int J LCA 9: 114-121.
- Curzons AD, Jimenez-Gonzalez C, Duncan AL, Constable DJC, Cunningham VL (2007) Int J LCA 12: 272-280.
- Sheldon RA (2005) Green solvents for sustainable organic synthesis: state of the art. Green Chem 7(5): 267-278.
- FDA Consumer Health Information (2009) Hidden Risks of Erectile Dysfunction Treatments Sold Online.
- Jer-Huei Lin (2006) Identification of a Sildenafil Analogue Adulterated in Two Herbal Food Supplements. Journal of Food and Drug Analysis 14(3): 260-264.
- Hakan Goker (2012) Chromatographic Separation and Identification of Sildenafil and Yohimbine Analogues Illegally Added in Herbal Supplements. Biochemistry, Genetics and Molecular Biology.
- (2012) Four Fake Doctors Jailed. The Daily Star.
- (2012) Indian herbal 'Sandhi Sudha' floods Bangladesh markets.
- (2013) Indications and Clinical Use, Product Monograph of VIAGRA (sildenafil citrate).
- PATENTS- EP 1002798, EP 1779852, EP 0916675, US6066735, US6204383, US2010048897, WO0119827, WO122918, and WO2004072079.






























