New Benzothiazole-Thiazolidinone hybrids containing Phthalimidoxy and Ethoxyphthalimide: Design, Synthesis and Pharmacological Assay
Prakash Prajapat1* and Ganpat L Talesara2
1Department of Chemistry, Ganpat University, India
2Department of Chemistry, MLS University, India
Submission: March 07, 2018; Published: March 20, 2018
*Corresponding author: Prakash Prajapat, Department of Chemistry, Ganpat University, Mehesana-384012, Gujarat, India; Email: prajapatprakash11@yahoo.in
How to cite this article: Prakash P, Ganpat L T. New Benzothiazole-Thiazolidinone hybrids containing Phthalimidoxy and Ethoxyphthalimide: Design, Synthesis and Pharmacological Assay. 005 . Organic & Medicinal Chem IJ. 2018; 5(5): 555673. DOI: 10.19080/OMCIJ.2018.05.555673
Abstract
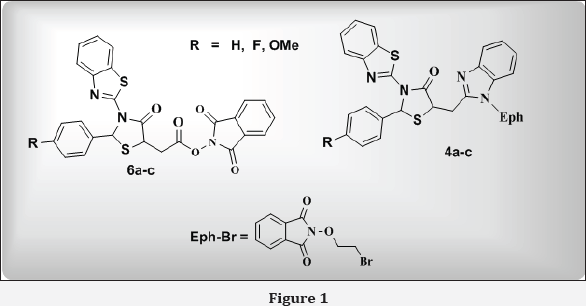
In an endeavor to find a new class of antimicrobial agents, a series of 2-(2-{2-[3-benzothiazol-2-yl-2-(4-substituted-phenyl)-4-oxo-thiazolidin-5- ylmethyl]-benzoimidazol-1-yl}-ethoxy)-isoindole-1,3-dione (4a-c) and 1,3-dioxoisoindolin-2-yl-2-(3-(benzo[d]thiazol-2-yl)-2-(4- substituted phenyl)-4-oxothiazolidin-5-yl) acetate (6a-c) have been designed and synthesized from benzothiazol-2-yl-(4-substituted-benzylidene)-amine (1a-c). Structural elucidation of the synthesized compounds is accomplished by elemental analysis, spectral data (FT-IR, 1H-NMR & mass) and chemical tests. These have been assayed for their antimicrobial activity against pathogenic S. aureus, S. pyogenus, E. coli, P. aeruginosa (bacterial species) and A. niger, C. albicans, A. clavatus (Fungal species) (Figure 1). Some of hybrids were found to be equipotent or more potent than the reference drugs.
Keywords: Biological activity, Benzothiazole, Thiazolidinone, Phthalimidoxy, Ethoxyphthalimide
Introduction
Medicinal and pharmaceutical chemistry is a scientific discipline at the interaction of chemistry and pharmacology involved with designing, synthesizing and developing pharmaceutical drugs. The development of new drug and pharmaceuticals is currently a critical and challenging issue to the researchers and pharmaceutical industry. A wide range of synthetic and medicinal properties shown by heterocyclic hybrids inspired organic and medicinal chemists to pursue the synthesis of newer motifs and evaluate their pharmacophoric properties [1-6]. Modifications on the benzothiazole scaffold have resulted in a huge number of compounds having diverse pharmacological activities [7,8]. Thus, synthesis and pharmacological activities of benzothiazole derivatives have long been focused in the field of medicinal and pharmaceutical chemistry especially 2-substituted benzothiazole derivatives [9-11].
Some important and clinically used drugs having benzothiazole ring in their structures are Riluzole, Thioflavin, Pittsburgh compound B, Ethoxzolamine, Pramipexole, Dimazole, Flutemetamol and Dithiazanine Iodide (Figure 2). Thiazolidinone and its derivatives is one of the significant heterocyclic ring system has therapeutic importance and when hybridize with other heterocyclic rings produce broad range of bioactivities such as antibacterial, anti-inflammatory activity etc [12-14]. Similarly, benzimidazoles are chemically and biologically effective target molecules, and have been studied extensively for their DNA sequence recognition properties.

Anticancer, antibacterial, antifungal properties are the principal bioactivities tested by N-hydroxy substituted moieties (-N-O- linkage), signifying that they beneficial for several types of cancer and other infectious diseases. Various aminooxy moieties have been studied by Berger for their ability to inhibit the growth of the malaria parasite Plasmodium falciparum in vitro. In view of these findings and in continuation of our interest in the synthesis of new phthalimidoxy and ethoxyphthalimide containing heterocyclic framework, the plan was to design and synthesize a new class of hybrid molecule in which all of the above moieties are present with the hope to achieve enhanced pharmacological activity [15-21].
Results and Discussions
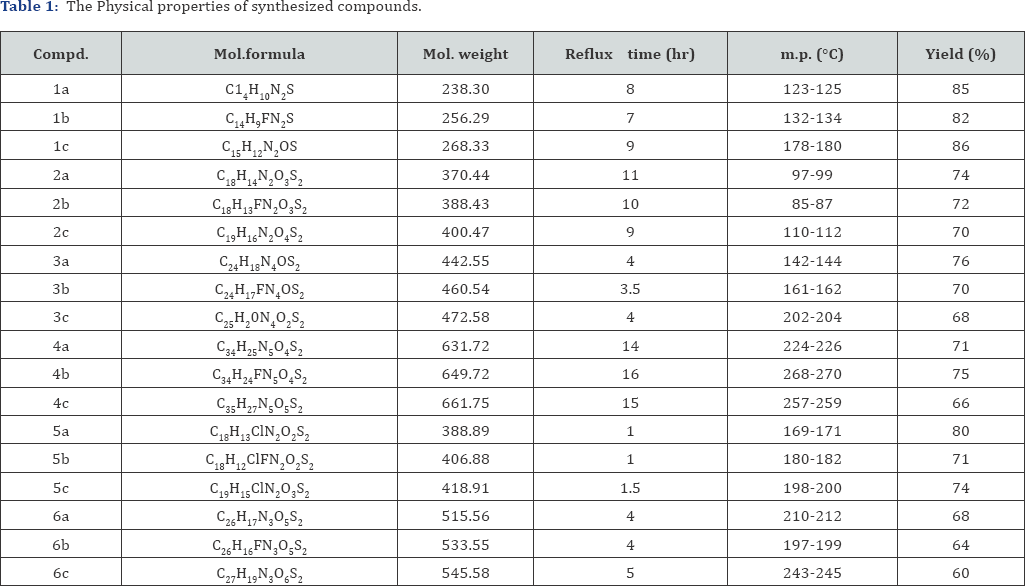
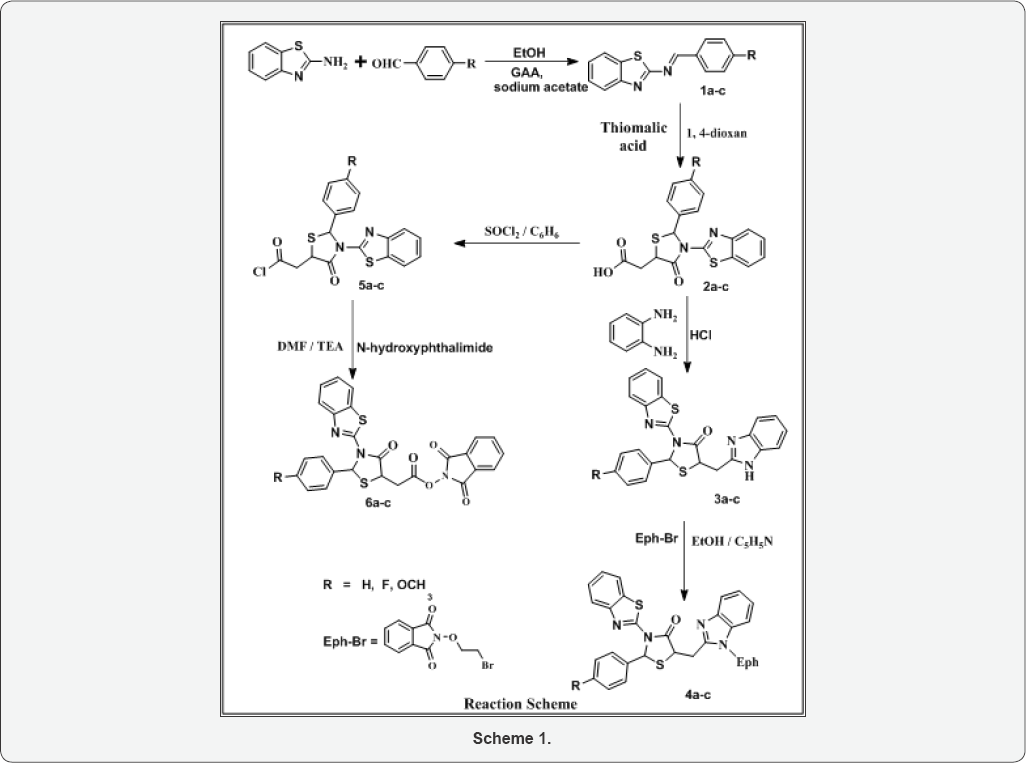
The synthetic procedures adopted to obtain the target hybrids are outlined in reaction scheme. Benzothiazol-2-yl-(4- substituted-benzylidene)-amine (1a-c) have been synthesized by the condensation reaction of 2-aminobenzothiazole with various arylaldehyde. Additionally, when 1a-c were reacted with thiomalic acid, it gave [3-Benzothiazol-2-yl-2-(4-substituted- phenyl)-4-oxo-thiazolidin-5-yl]-acetic acid (2a-c). The IR Spectrum of the compound 2a shows bands 2745 cm-1 due to OH, stretching of COOH group, as expected for the formation of compound 2a, which was confirmed by 1H NMR spectrum. In addition to it, we found a peak at 5 10.52 singlets, which showed the present of OH group. Further the reaction involving synthesis of 5-(1H-Benzoimidazol-2-ylmethyl)-3-benzothiazol-2-yl-2- (4-substituted-phenyl)-thiazolidin-4-one (3a-c) took place by reacting [3-Benzothiazol-2-yl-2-(4-substituted-phenyl)-4-oxo- thiazolidin-5-yl]-acetic acid (2a-c) with o-phenylenediamine. Formation of 3a was confirmed by NH stretching at 3342 cm'1 in IR region and characteristic peak at 5 6.44 in 1H NMR for NH group. Compound 3a were reacted with bromethoxy phthalimide to gave compound 4a as shown by new two triplet peak of 3.46 (t, O-CH2 ) and 3.18 of (t, N-CH2 ) groups in 1H NMR spectra. It also elucidated by disappearance of NH peak in IR and NMR region. In another route, when compounds 2a-c have been refluxed with thionyl chloride in benzene to give corresponding derivatives 5a- c. Formations of compounds were confirmed by disappearance of IR band of -COOH group and appearance of new IR band at 735-710 cm-1 due to formation of C-Cl bond. Here the Cl atom of -CH2-CO-Cl is replaced by N-hydroxyphathalimide to furnish final products (6a-c). Formation of these products was confirmed by CO-N-CO stretching around at 1685-1678 cm-1 in IR spectrum and characteristics signals appeared at 5 3.88-3.97 for -CH2COO group. The mass spectrum also supports the proposed structure by viewing molecular ion peaks of final products. Addition confirmation of phthalimidoxyl group attachment was achieved by usual chemical test including fluorescence formation (Scheme 1) (Table 1).
Experimental
General
All chemicals were commercially procured and were used without further purification. Melting points were determined in open capillary tube and are therefore uncorrected. Purity of synthesized compounds was checked by TLC using silica gel-G plates, n-hexane - ethyl acetate as developing solvent and the spots were exposed in an UV light. Fourier transform infrared (FT-IR) spectra were recorded with a Bruker spectrometer model alpha and NMR was recorded on a Bruker DRX-400 MHz spectrometer with dimethylsulfoxide DMSO-d6/CDCl3 as solvent using tetramethylsilane (TMS) as an internal standard. The mass spectra were recorded on water Q-TOF, Micromass (ES) model. Elemental analysis was done on "Heraeus Rapid Analyser” Structures of synthesized compounds were characterized by using IR, NMR, Mass and Elemental analysis.
Synthesis of compound (1a)
2-aminobenzothiazole (0.01 mol) was added to a solution of benzaldehyde (0.012 mol) in dry ethanol (40 mL) in a round bottom flask, presence acetic acid as a catalyst and 4-5 drops of fused sodium acetate and refluxed for 8 hr. At the end of the reaction the solvents were partially evaporated then poured into cold water. The precipitates were collected by filtration, washed with ether, dried and recrystallized from the ethanol. Compounds 1b and 1c were also prepared by similar method with minor changes in reaction conditions.
Benzothiazol-2-yl-benzylidene-amine (1a)
IR (vmax, cm-1): 3030 (Ar-H), 1610 (C=N str.), 1450 (C=C str. Ar) 1285 (C-N), 705 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 6.89-7.29 (m, 9H, Ar-H), 8.20 (s, 2H, N=CH-Ar).
Benzothiazol-2-yl-(4-fluoro-benzylidene)-amine (1b)
IR (vmax, cm’1): 3015 (Ar-H), 1624 (C=N str.), 1435 (C=C str. Ar) 1264 (C-N), 699 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 6.88-7.26 (m, 8H, Ar-H), 8.22 (s, 2H, N=CH-Ar).
Benzothiazol-2-yl-(4-methoxy-benzylidene)-amine (1c)
IR (vmax, cm-1): 3045 (Ar-H), 2895 (C-H str.),1622 (C=N str.), 1455 (C=C str. Ar) 1282 (C-N), 698 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 6.89-7.28 (m, 8H, Ar-H), 8.42 (s, 2H, N=CH-Ar), 2.82 (s, 3H, OCH3).
Synthesis of compound (2a)
A mixture of equimolar mixture of compound 1a (0.01 mol) and mercaptosuccinic acid (0.01 mol) in dioxan (30 ml) with a pinch of anhydrous ZnCl2 was refluxed for 11 hrs on a water bath. The reaction mixture was left to cool at room temperature. The solid product so formed was collected and crystallized from methanol. Compounds 2b and 2c were also prepared by similar method with minor changes in reaction conditions.
(3-Benzothiazol-2-yl-4-oxo-2-phenyl-thiazolidin-5- yl)-acetic acid (2a)
IR (vmax, cm-1): 3050 (C-H str., Ar-H), 2745 (COOH str.), 1735 (str., C=O acid str.), 1720 (C=O str.) 1589 (C=N str.), 1410 (C=C str. Ar) 1255 (C-N), 688 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 10.52 (s, 2H, COOH), 6.92-7.42 (m, 9H, Ar-H), 5.88 (s, 1H, CH), 3.73 (t, 2H, CH2), 2.63 (d, 1H, CH); LCMS: m/z 370 [M+]; Anal. calcd. For C18H14N2O3S2: C, 58.36; H, 3.81; N, 7.56; Found: C, 58.45; H, 3.90; N, 7.67%.
[3-Benzothiazol-2-yl-2-(4-fluoro-phenyl)-4-oxo- thiazolidin-5-yl]-actic acid (2b)
IR (vmax, cm-1): 3042 (C-H str., Ar-H), 2738 (COOH str.), 1736 (str., C=O acid str.), 1715 (C=O) 1590 (C=N str.), 1412 (C=C str. Ar) 1256 (C-N), 699 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 10.52 (s, 2H, COOH), 6.92-7.45 (m, 9H, Ar-H), 5.89 (s, 1H, CH), 3.71 (t, 2H, CH2), 2.62 (d, 1H, CH); LCMS: m/z 388 [M+]; Anal. calcd. For C18H13FN2O3S2: C, 55.66; H, 3.37; N, 7.21; Found: C, 56.79; H, 3.46; N, 7.12%.
[3-Benzothiazol-2-yl-2-(4-methoxy-phenyl)-4-oxo- thiazolidin-5-yl]-acetic acid (2c)
IR (vmax, cm-1): 3025 (C-H str., Ar-H), 2890 (C-H str.), 2732 (COOH str.), 1738 (C=O acid str.), 1715 (C=O, str.) 1592 (C=N str.), 1415 (C=C str. Ar) 1254 (C-N), 701 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 10.52 (s, 2H, COOH), 6.92-7.44 (m, 9H, Ar-H), δ 5.89 (s, 1H, CH), 3.71 (t, 2H, CH2), 2.63 (d, 1H, CH), 2.81 (s, 3H, OCH3); LCMS: m/z 400 [M+]; Anal. calcd. For C19H16N2O4S2: C, 56.98; H, 4.03; N, 7.00; Found: C, 56.90; H, 4.38; N, 7.15%.
Synthesis of compound (3a)
A mixture of o-phenylenediamine (0.01 mol), compound (2a) (0.01 mol) and 4N HCl (100 mL) was refluxed for 4h. The reaction mixture was then cooled and neutralized with dil NaOH. The precipitate separated out was filtered, washed with cold water and dried. The product was recrystallized from hot aq. ethanol to obtain the pure compound. Compounds 3b and 3c were also prepared by similar method with minor changes in reaction conditions.
5-(1H-Benzoimidazol-2-ylmethyl)-3-benzothiazol-2- yl-2-phenyl-thiazolidin-4-one (3a)
IR (vmax, cm-1): 3342 (N-H), 3025 (C-H str., Ar-H), 2890 (C-H str.), 1715 (C=O str.) 1605 (C=N str.), 1405 (C=C str. Ar) 1260 (C-N), 702 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 6.89-7.99 (m, 13H, Ar-H), 6.44 (s, 1H, NH), 5.85 (s, 1H, CH), 4.14 (t, 2H, CH2), 2.35 (d, 1H, CH); LCMS: m/z 442 [M+]; Anal. calcd. For C24H18N4OS2: C, 65.13; H, 4.10; N, 12.66; Found: C, 65.35; H, 4.38; N, 12.52%.
5-(1H-Benzoimidazol-2-ylmethyl)-3-benzothiazol-2- yl-2-(4-fluoro-phenyl)-thiazolidin-4-one (3b)
IR (vmax, cm-1): 3339 (N-H), 3025 (C-H str., Ar-H), 2890 (C-H str.), 1715 (C=O str.) 1610 (C=N str.), 1462 (C=C str. Ar) 1258 (C-N), 698 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 6.90-7.98 (m, 12H, Ar-H), 6.45 (s, 1H, NH), 5.85 (s, 1H, CH), 4.15 (t, 2H, CH2), 2.39 (d, 1H, CH); LCMS: m/z 460 [M+]; Anal. calcd. For C24H17FN4OS2: C, 62.59; H, 3.72; N, 12.17; Found: C, 62.72; H, 3.98; N, 12.01%.
5-(1H-Benzoimidazol-2-ylmethyl)-3-benzothiazol-2- yl-2-(4-methoxy-phenyl)-thiazolidin-4-one (3c)
IR (vmax, cm-1): 3322 (N-H), 3025 (C-H str., Ar-H), 2890 (C-H str.), 1715 (C=O str. ) 1608 (C=N str.), 1457 (C=C str. Ar) 1264 (C-N), 695 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 6.92-7.98 (m, 12H, Ar-H), 6.44 (s, 1H, NH), 5.85 (s, 1H, CH), 4.14 (t, 2H, CH2), 2.78 (s, 3H, OCH3), 2.38 (d, 1H, CH); LCMS: m/z 472 [M+]; Anal. calcd. For C25H20N4O2S2: C, 63.54; H, 4.27; N, 11.86; Found: C, 63.42; H, 4.39; N, 11.65%.
Synthesis of compounds (4a):
A mixture of compound 3a (0.01 mol) and Bromoethoxyphthalimide (0.01 mol) in ethanol (25 ml) and pyridine (0.01 mol) was refluxed for 14 hr in a round bottom flask. It was cooled to room temperature, and the mixture was slowly poured on to crushed ice with constant stirring. Solid obtained was filtered and washed cooled water. It was recrystallized from ethanol. Compounds 4b and 4c were also synthesized by similar method with minor changes in reaction conditions.
2-{2-[2-(3-Benzothiazol-2-yl-4-oxo-2-phenyl- thiazolidin-5-ylmethyl)-benzoimidazol-1-yl]-ethoxy}- isoindole-1,3-dione (4a)
IR (vmax, cm'1): 3056 (Ar-H), 2841 (C-H str.), 1715 (C=O str.), 1695 (CO-IN-CO), 1599 (C=N), 1455 (C=C, Ar), 1360 (N-O), 1236 (C-N), 1027 (C-O), 688 (C-S-C); *H NMR (400 MHz, DMSO-d6): 5 6.98- 8.02 (m, 17H, Ar-H), 6.56 (s, 1H, NH), 5.92 (s, 1H, CH), 4.02 (t, 2H, CH2), 3.46 (t, 2H, OCH2 ), 3.18 (t, 2H, NCH2), 2.38 (d, 1H, CH); LCMS: m/z 631 [M+]; Anal. calcd. For C25H20N4O2S2: C, 64.54; H, 3.99; N, 11.09; Found: C, 64.42; H, 4.39; N, 11.52%.
2-(2-{2-[3-Benzothiazol-2-yl-2-(4-fluoro-phenyl)- 4-oxo-thiazolidin-5-ylmethyl]-benzoimidazol-1-yl}- ethoxy)-isoindole-1,3-dione (4b)
IR (vmax, cm’1): 3055 (Ar-H), 2835 (C-H str.), 1705 (C=O str.), 1690 (CO-N-CO), 1608 (C=N), 1449 (C=C, Ar), 1362 (NO), 1240(C-N), 1038 (C-O), 692 (C-S-C); 1H NMR (400 MHz, DMSO-d6): 5 6.98-8.03 (m, 16H, Ar-H), 6.44 (s, 1H, NH), 5.99 (s, 1H, CH), 4.08 (t, 2H, CH2), 3.48 (t, 2H, OCH2 ), 3.22 (t, 2H, NCH2), 2.34 (d, 1H, CH); LCMS: m/z 649 [M+]; Anal. calcd. For C-,H-nN,O-S-: C, 62.85; H, 3.72; N, 10.78; Found: C, 62.75; H, 3.89; 25 20 4 2 2 N, 11.05%.
2-(2-{2-[3-Benzothiazol-2-yl-2-(4-methoxy-phenyl)- 4-oxo-thiazolidin-5-ylmethyl]-benzoimidazol-1-yl}- ethoxy)-isoindole-1,3-dione (4c)
IR (vmax, cm-1): 3050 (Ar-H), 2842 (C-H str.), 1722 (C=O str.), 1699 (CO-IN-CO), 1598 (C=N), 1457 (C=C, Ar), 1358 (N-O), 1231 (C-N), 1030 (C-O), 689 (C-S-C); 1H NMR (400 MHz, DMSO-d6): 5 6.98- 8.11 (m, 16H, Ar-H), 6.52 (s, 1H, NH), 5.97 (s, 1H, CH), 4.19 (t, 2H, CH2), 3.44 (t, 2H, OCH2 ), 3.19 (t, 2H, NCH2), 2.83 (s, 3H, OCH3), 2.38 (d, 1H, CH); LCMS: m/z 661 [M+]; Anal. calcd. For C-,H-nN,O-S-: C, 63.52; H, 4.11; N, 10.58; Found: C, 63.42; H, 4.39;25 20 4 2 2N, 10.65%.
Synthesis of compounds (5a):
A solution of compound 2a (0.01 mol) in benzene (30 mL) and thionyl chloride (0.02 mol) was refluxed for 1 hr on water bath. Excess of thionyl chloride was removed under reduced pressure. On cooling, solid precipitate obtained, was filtered, dried and crystallized from ethanol. Likewise other compounds 3.I.Vb and 3.I.Vc were also synthesized.
2-(3-(Benzo[d]thiazol-2-yl)-4-oxo-2- phenylthiazolidin-5-yl)acetyl chloride (5a)
IR (vmax, cm-1): 3032 (Ar-H), 2869 (C-H str.), 1765 (COCl), 1708 (C=O str.), 1565 (C=N), 1422 (C=C, Ar), 1210 (C-N), 694 (C-S-C) 710 (C-Cl); 1H NMR (400 MHz, DMSO-d6): δ 6.98-7.48 (m, 9H, Ar- H), 5.97 (s, 1H, CH), 3.96 (t, 2H, CH2), 2.58 (d, 1H, CH); LCMS: m/z 388 [M+], 390 [M+2]+; Anal. calcd. For C18H13ClN2O2S2: C, 55.59; H, 3.37; N, 7.20; Found: C, 55.81; H, 3.19; N, 7.45%.
2-(3-(Benzo[d]thiazol-2-yl)-2-(4-fluorophenyl)-4- oxothiazolidin-5-yl)acetyl chloride (5b)
IR (vmax, cm-1): 3035 (Ar-H), 2872 (C-H str.), 1775 (COCl), 1712 (C=O str.), 1579 (C=N), 1420 (C=C, Ar), 1225 (C-N), 697 (C-S-C) 728 (C-Cl); 1H NMR (400 MHz, DMSO-d6): δ 6.96-7.44 (m, 8H, Ar- H), 6.01 (s, 1H, CH), 3.92 (t, 2H, CH2), 2.64 (d, 1H, CH); LCMS: m/z 406 [M+], 408 [M+2]+ ; Anal. calcd. For C18H12ClFN2O2S2: C, 53.13;
H, 2.97; N, 6.67; Found: C, 53.42; H, 2.39; N, 6.55%.
2-(3-(Benzo[d]thiazol-2-yl)-2-(4-methoxyphenyl)-4- oxothiazolidin-5-yl)acetyl chloride (5c)
IR (vmax, cm-1): 3038 (Ar-H), 2884(C-H str.), 1772 (COCl), 1705 (C=O str.), 1580 (C=N), 1415 (C=C, Ar), 1220 (C-N), 698 (C-S-C) 735 (C-Cl); 1H NMR (400 MHz, DMSO-d6): δ 6.97-7.46 (m, 8H, Ar-H), 5.91 (s, 1H, CH), 3.85 (t, 2H, CH2), 2.79 (s, 3H, OCH3), 2.52 (d, 1H, CH); LCMS: m/z 418 [M+], 420 [M+2]+ ; Anal. calcd. For C19H15ClN2O3S2: C, 54.47; H, 3.61; N, 6.69; Found: C, 54.42; H, 4.89; IN, 6.85%.
Synthesis of compounds (6a):
To a solution of compound 5a (0.01 mol) in dry DMF (30 mL), N-hydroxyphthalimide (0.01 mol) and TEA (0.01 mol) was added. The reaction mixture was stirred at room temperature for one hr than it was refluxed for 4 hr. Excess of solvent was removed under reduced pressure and solid product obtained was filtered, dried and recrystallized from methanol. Compounds 6b and 6c were synthesized by the similar method with minor modification in mole ratio of reagents by changing in reflux time. The observed physical properties presented in Table 1.
I, 3-Dioxoisoindolin-2-yl-2-(3-(benzo[d]thiazol-2-yl)- 4-oxo-2-phenylthiazolidin-5-yl)acetate (6a)
IR (vmax, cm-1): 3020 (Ar-H), 2887 (C-H str.), 1708 (C=O str.), 1672 (CO-N-CO), 1598 (C=N), 1480 (C=C, Ar), 1341 (N-O), 1215 (C-N), 669 (C-S-C); 1H NMR (400 MHz, DMSO-d6): δ 6.96-7.78 (m, 13H, Ar-H), 5.68 (s, 1H, CH), 3.88 (t, 2H, CH2), 2.42 (d, 1H, CH); LCMS: m/z 515 [M+]; Anal. calcd. For C26H17N3O5S2: C, 60.57; H, 3.32; N, 8.15; Found: C, 60.42; H, 3.48; N, 8.37%.
1,3-Dioxoisoindolin-2-yl-2-(3-(benzo[d]thiazol-2-yl)- 2-(4-fluorophenyl)-4-oxothiazolidin-5-yl)acetate (6b)
IR (vmax, cm-1): 3017 (Ar-H), 2868 (C-H str.), 1710 (C=O str.), 1678 (CO-IN-CO), 1574 (C=N), 1488 (C=C, Ar), 1340 (N-O), 1205 (C-N), 671 (C-S-C); H NMR (400 MHz, DMSO-d6): 5 6.94-7.74 (m, 12H, Ar-H), 5.66 (s, 1H, CH), 3.94 (t, 2H, CH2), 2.45 (d, 1H, CH); LCMS: m/z 533 [M+]; Anal. calcd. For C26H16FN3O5S2: C, 58.53; H, 3.02; N, 7.88; Found: C, 58.42; H, 3.39; N, 7.65%).
1,3-Dioxoisoindolin-2-yl-2-(3-(benzo[d]thiazol-2-yl)- 2-(4-methoxyphenyl)-4-oxothiazolidin-5-yl)acetate (6c)
IR (vmax, cm-1): 3015 (Ar-H), 2874 (C-H str.), 1707 (C=O str.), 1685 (CO-IN-CO), 1576 (C=N), 1485 (C=C, Ar), 1345 (N-O), 1212 (C-N), 665 (C-S-C); 1H NMR (400 MHz, DMSO-d6): 5 6.97-7.79 (m, 12H, Ar-H), 5.67 (s, 1H, CH), 3.97 (t, 2H, CH2), 2.88 (s, 3H, OCH3), 2.42 (d, 1H, CH); LCMS: m/z 545 [M+]; Anal. calcd. For C H N OCS,: C, 59.44; H, 3.51; N, 7.70; Found: C, 59.38; H, 3.59;27 19 3 6 2 N, 7.85%.
Pharmacology
S. pyogenes Synthesized molecular hybrids 4a-c and 6a-c have been examined for their in-vitro antibacterial activity against two gram-positive bacteria viz. Staphylococcs aureus (MTCC 96) and S. pyogenes (MTCC 443) and two gram-negative bacteria like Escherichia coli (MTCC 442) and Pseudomonas aeruginosa (MTCC 441) by using ampicillin as the reference antibacterial drug. In- vitro antifungal activity was also performed against three fungal species-Candida albicans (MTCC 227), Aspergillus niger (MTCC 282) and Aspergillus clavatus (MTCC 132 3) where griseofulvin was used as the reference antifungal drug. The minimal inhibitory concentration (MICs, μg/mL) of the synthesized hybrids was determined by the broth micro-dilution method. The results of this investigation are presented in Table 2. The test molecular hybrids showed significant activity when compared to standard drugs.

MICs (μg/mL) values in bold letters indicate that the synthesized hybrid molecules are comparatively equipotent or more potent than the reference drugs.
Antibacterial Activity
The newly synthesized derivatives were assayed for their antibacterial activity against bacterial strain E.coli and P. aeruginosa, S. aureus and S. pyogenus. Compound 4c showed strong activity against E. coli whereas the compounds 6a and 6c were similar MIC values as compared to ampicillin against E. coli species. All synthesized compounds showed good activity against P. aeruginosa when compare to ampicillin. Compounds 4b, 4c and 6c exhibited strong activity against S. aureus whereas compound 4a showed better activity than other compounds against gram positive bacteria S. aureus. Compound 4a showed similar MIC value against S. pyogenus whereas other compounds showed moderate activity as compared to ampicilin.
Antifungal Activity
The synthesized compounds were evaluated for their antifungal activity against fungal species A. niger, C. albicans and A. clavatus. The synthesized derivative 4a showed strong activity against C. albicans fungal strain whereas the compound 6a exhibited similar activity. Compound 6c showed strong activity against A. clavatus when compared to greseofulvin (Table 2).
Conclusion
A series of benzothiazole based molecular hybrids were synthesized, and assayed for their pharmacological activity with the aim of discovering innovative structure leads serving as potent antibacterial, antifungal agents. Out of six compounds screened four compounds i.e., 4a, 4b, 4c and 6c showed good antibacterial activity against most of bacterial and some of fungal species when compared to reference drugs. Hence, conclusions can be drawn that the synthesized compounds are better antibacterial agents as compared to antifungal and they can be developed as potent chemotherapeutic agents. This synthetic approach opens the door to the synthesis of a variety of novel molecular hybrids in future for development of new antibacterial agents.
Acknowledgement
I thankful to chemistry staff members of the Mehesana Urban Institute of Sciences, Ganpat University, Gujarat for their kind support and Head, Department of Chemistry, ML Sukhadia University, Udaipur (Rajasthan) for providing laboratory facilities, the Director, NFDD Rajkot, India for providing spectral and analytical data.
References
- Prajapat P, Vaghani H, Agarwal S, Talesara GL (2017) Synthetic and Medicinal Chemistry in Drug Discovery: Needs for Today. Ann Med Chem Res 3(1): 1021-1022.
- Prajapat P, Agarwal S, Talesara GL (2017) Significance of Computer Aided Drug Design and 3D QSAR in Modern Drug Discovery. J Med Org Chem 1(1): 1.
- Prajapat P (2017) Utility of Drug Discovery in Medicinal and Organic Chemistry. Mod Chem Appl 5(4): 1-2.
- Prakash P (2018) Importance of Benzothiazole Motif in Modern Drug Discovery: Introduction. Mod Appro Drug Des 1(4): 1-2.
- Prajapat P (2018) Some Important Applications of Recent Advances in Applied Chemical Science. Mod Chem Appl 6(1): 1-2.
- Prajapat P (2018) Role of Combinatorial, Medicinal & Biological chemistry in drug discovery development: An Introduction. Arc Org Inorg Chem Sci 1(1).
- Agarwal S, Kalal P, Gandhi D, Prajapat P (2017) Thiazole containing Heterocycles with CNS activity. Curr Drug Discov Technol.
- Patel RV, Park SW (2015) Catalytic N-formylation for synthesis of 6-substituted-2-benzothiazlylimino-5-piperazinyl-4-thiazolidinone antimicrobial agents. Res Chem Intermed 41(8): 5599-5609.
- Mahtab R, Srivastava A, Gupta N, Kushwaha SK, Tripathi, A (2014) Synthesis of novel 2-benzylbenzo[d] thiazole-6-sulfonamide derivatives as potential anti-inflammatory agent. J Chem Pharma Sci 7: 34-38.
- Hazra K, Nargund LVG, Rashmi P, Chandra JNNS, Nandha B, et al. (2012) Synthesis and comparative study of anti-mycobacterium activity of a novel series of fluoronitrobenzothiazolopyrazoline regioisomers. Arch Pharm Chem Life Sci 345(2): 137-146.
- Mir F, Shafi S, Zaman MS, Kalia NP, Rajput VS, et al. (2014) Sulfur rich 2-mercaptobenzothiazole and 1,2,3-triazole conjugates as novel antitubercular agents. Eur J Med Chem 76: 274-283.
- Shrivastava J, Dubey P, Singh S, Bhat HR, Kumawat MK, et al. (2015) Discovery of novel 1,3,5-triazine-thiazolidine-2, 4-diones as dipeptidyl peptidase-4 (DPP-4) inhibitor targeting S1 pocket for the treatment of type 2 diabetes along with antibacterial activity. RSC Adv 5(19): 1409514102.
- Barros CD, Amato AA, Oliveira TB, Iannini KBR, Silva AL, et al. (2010) Synthesis and anti-inflammatory activity of new arylidene- thiazolidine-2, 4-dione as PPAR ligands. Bioorg Med Chem 18(11): 3805-3811.
- Prajapat P, Yogi P, Talesara GL (2015) Synthesis of Biological Significant New 1-(1, 3-benzoxazol-2-yl)guanidine Derivatives. Journal of Chemistry and Chemical Sciences 5(12): 670-681.
- Prajapat P, Talesara GL (2016) Synthesis and Anti-inflammatory Screening of Some Mono and Bis-Alkoxyphthalimide Linked Benzimidazole and their Quinazoline and Pyrimidine Derivatives. J Heterocyclic Chem 53(5): 1603-1610.
- Prajapat P, Kumawat M, Kherodiya B, Talesara GL (2016) An expedient synthesis and antimicrobial evaluation of ethoxyphthalimido derivatives of pyrimido [4, 5-e] pyrimidine analogues from 1- (1H-benzimidazol-2-l)guanidine. J Indian Chem Soc 93: 539-544.
- Kumawat M, Kherodiya B, Prajapat P, Talesara GL (2015) Synthesis of alkoxyphthalimide derivatized oxoimidazolidinyl oxazolo/thiazolo dihydropyrimidine and oxoimidazolidinyl tetrahydropyrimidine via common Schiff base intermediate and evaluation of their antibacterial activity. Indian J Chem 54(1): 117-127.
- Prajapat P, Rathore KK, Hussain N, Yogi PP, Talesara GL (2015) Synthesis of novel pyrimidines, pyrimidopyrimidines and their oxygen substituted hydroxylamine derivatives as potential pharmacological interest. Iranian Journal of Organic Chemistry 7(3): 1605-1612.
- Kherodiya B, Prajapat P, Kumawat M, Talesara GL (2015) Synthesis and antimicrobial evaluation of bis imidazolidinone assembled dihydropyridine ethoxyphthalimide derivatives. Iranian Journal of Organic Chemistry 7(4): 1661-1668.
- Prajapat P, Rathore KK, Gandhi D, Agarwal S, Hussain N, et al. (2016) A Facile Synthesis of Biologically Significant 2-(1,3-benzothiazol- 2- ylimino)-1,3-thiazolidin-4-one/3-(1,3-benzothiazol-2-yl)-2- thioxoimidazolidin-4-on Analogues from 1-(1,3-benzothiazol-2-yl) thiourea and their Alphahydroxylamine Derivatives. Iranian Journal of Organic Chemistry 8(2): 1795-1801.
- Hussain N, Ahmed M, Khan S, Joshi A, Yogi P, et al. (2017) One-pot Synthesis of Bis[1-N,7-N-diethoxyphthalimido-4,4'-{3,5-dimethyl- 4-(4-N,N-dimethyl Aminophenyl)-4,7-dihydro-1H-dipyrazolo[3,4- b;4',3'-e]Pyridin-8-yl}-phenyl] Compounds and Their Antiviral Evaluation Against Cytomegalovirus (CMV) and Varicella-zoster Virus (VZV) in Human Embryonic Lung (HEL) Cells. J Heterocyclic Chem 54(2): 993-998.






























