CCK1 antagonists: Design, Synthesis and Evaluation of N-Substituted Isobuyl-5-Hydroxy-5-Phenyl-Pyrrol-2- Ones as Adjunct to Opiates
Eric Lattmann1*, Jintana Sattayasai2, Ramesh Narayanan3, Julian Benyamen1, PN Balaram4 and Pornthip Lattmann4
1School of Life and Health Sciences, Aston University, England
2Department of Pharmacology, Faculty of Medicine, Khon Kaen University, Thailand
3Department of Medicine, University of Tennessee Health Science Center, USA
4PNB Vesper Life Science PVT, India
Submission: February 26, 2018; Published: March 09, 2018
*Corresponding author: Eric Lattmann, Aston School of Pharmacy, Aston University, Aston Triangle, England; Tel: +44-[0)1212043980; Fax: +44- (0)1213590733; Email: e.lattmann@aston.ac.uk
How to cite this article: Eric L, Jintana S, Ramesh N, Julian B, PN Balaram, Pornthip L. CCK1-antagonists: Design, Synthesis and Evaluation of N-Substituted Isobuyl-5-Hydroxy-5-Phenyl-Pyrrol-2-Ones as Adjunct to Opiates. Organic & Medicinal Chem IJ. 2018; 5(5): 555672. DOI: 10.19080/OMCIJ.2018.05.555672
Abstract
Arylated 5-hydroxy-pyrrol-2-ones were prepared in 2 synthetic steps from muco- chloric acid and were optimised as CCK selective ligands using radiolabelled binding assays. A potent CCK selective ligand was identified ( PNB-081: CCKμ 20 nM) as part of systematic SAR optimisation. The antagonism was confirmed for the ligands by using isolated tissue preparations with CCK8S. The cholecystokinin antagonist PNB-081 potentiated the analgesic effect of morphine and reversed opiate tolerance in mice from doses >1 mg/kg by oral administration.
Keywords: Phenyl-Pyrrolone; CCK Antagonist; Cholecystokinin; Analgesic; Opiate Adjunct
Introduction
In terms of cholecystokinin-physiology, CCK8 is the most common peptide hormone, which is extensively found throughout the gastrointestinal tract and is also widely distributed through the nervous system [1,2]. Originally, cholecystokinin was discovered to cause contractions of the gallbladder [3]. It was then rediscovered as pancreozymin, triggering the release of pancreatic enzymes. Finally, it was confirmed that both peptides are identical [4]. Cholecystokinin acts as a neuromodulator as well as gut hormone. CCK-ligands, agonists and antagonists have been extensively investigated as potential drug molecules [5]. Cholecystokinin antagonists have been extensively investigated as potential drug targets [6]. They were studied as growth inhibitors in certain forms of cancer, as anxiolytics, in the treatment of schizophrenia and satiety [7-10]. An agonist, the shortened CCK4 was found to induce panic in patients and the CCK2 receptor is known to mediate anxiety and panic attacks [11-13]. Cholecystokin in does cause proliferation in colon- and pancreatic cancer cell lines and therefore, CCK-antagonists were studied as growth factor inhibitors in certain forms of cancer.
Asperlicin was the first non-peptidal lead structure from nature and analogues thereof, were studied as CCK ligands [14,15]. Simplification of this lead structure by Merck led to Devazepide, a potent CCK1 selective cholecystokinin antagonist (Figure 1), containing a 1,4-benzodiazepine template and an indole moiety [16]. Proglumide was the first glutamic acid based agent, marketed as Milid for the treatment of ulcer. Lorglumide, a derivative of proglumide, is one of several CCK receptor antagonists and served as experimental standard. The indolyl amide of devazepide was replaced by a urea linkage and Merck's L-365,260 resulted in a CCK2 selective antagonist [17-20].
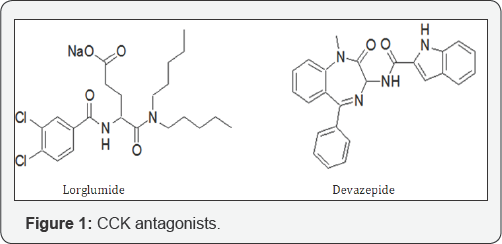
All structural optimisations did only partly address the main underlying problem with respect to poor pharmacokinetic properties, such as a low water solubility and very low membrane penetration, as a result of a large polar surface area of the molecules and a relatively high molecular weight. Again, having realized the poor pharmacokinetic properties these agents, a search for a completely novel, smaller template with a molecular weight <350, a log p about 3 and a polar surface area for membrane penetration of less that 100A, with no urea linkage was initiated. Aim of the drug discovery programme, initiated by PNB Vesper Life Sciences, was to systematically investigate and design the 2(5H)-furanone scaffold into a hydroxy-pyrrolone scaffold with ligands for both CCK pathways [21].
Molecular pain targets have been reviewed recently and the results are quite disappointing in terms of efficacy and FDA approval rate. Even, this review missed out on CCK antagonists and most importantly on a very positive report, publicised only in form of an abstract [22-24]. In summary in this study, devazepide at 5 mg was found very efficient in pain management as adjunct to strong opiates in a phase 2 trial carried out at leading UK pain research centres. Initial results for CCK antagonists of the pyrrolone scaffold were communicated in the area of cancer therapeutics and GI inflammation [25,26]. Here, a full biological evaluation of PNB-081 is reported in detail with respect to opiates and pain management [27].
Materials and Methods
Synthesis
The chemicals were obtained from Aldrich (Gillingham, UK) and Lancaster (Lancaster, UK). Atmospheric pressure chemical ionization mass spectroscopy (APC1), negative or positive mode, was carried out using a Hewlett-Packard 5989b quadrupole instrument (Vienna, Austria). Proton and Carbon NMR spectra were obtained on a Bruker AC 250 instrument (Follanden, Switzerland), operating at 250 MHz, calibrated with the solvent reference peak or TMS. IR spectra were plotted from KBr discs on a Mattson 300 FTIR Spectrometer. Melting points were recorded using a Stuart Scientific (Coventry, UK) apparatus and are uncorrected.
Synthesis of 3,4-dichloro-5-phenyl-5H-furan-2-one, Lactone A
Dry and powdered aluminium chloride (20g, 0.15mol) was added slowly to a mixture of mucochloric acid (16.9g, 0.1mol) and benzene / chlorobenzene (250 ml). The reaction mixture was stirred overnight. It was then poured into a mixture of 100g ice and 32 ml concentrated hydrochloric acid. The organic layer was separated by separating funnel and washed with 3 x 100 ml water. The combined organic layers were dried over magnesium sulphate and the solvent was removed under vacuum. The oily residue was crystallized in n-hexane. Yield = 70%; mp: 78-79oC; MS (APC1(+)): 195/197 (M+), 230/232 (M+1) m/z; *H NMR (CDCl3) 250 MHz: δ = 7.22-7.51 (m, 5H), 5.81 (s, 1H); 13C NMR (CDCl33):165.3, 152.2, 139.8, 130.5, 129.3, 128.5, 127.4, 127.2, 121.2, 83.5; IR (KBr-disc) υ max: 3445, 3074, 3035, 2959, 2056, 1768, 1630, 1499, 1457 1294, 1224, 1028, 910, 772, 705 cm-1.
3.4- Dichloro-5-(4-chloro-phenyl)-5H-furan-2-one, Lactone B
Yield = 69% mp: 76-78oC; MS (APC1(+)): 227/229/231 (M+1), 262/263/265 (M+) m/z; 1H NMR (CDCl3) 250 MHz: δ = 7.48 (m, 2H), 7.35 (m, 2H), 5.91 (s, 1H); 13C NMR (CDCl3) 165.3, 152.0, 136.6, 130.1, 129.6, 128.7, 121.3, 82.9; IR (KBr-d isc) u max: 3451, 3075, 2952, 2051, 1769, 1636, 1497, 1419, 1289, 1231, 1027, 927, 826, 748, 720 cm-1.
General Method
The relevant amine (2.3 times excess) was added to a solution of lactone A or B (0.7 mol) in ether (10 ml) and it was stirred on ice for 30 minutes, allowing to warm up to RT over time. The resultant mixture was poured into 5 ml of water and was separated by a separating funnel. The organic mixture was washed with water three times. The organic layer was dried over magnesium sulphate and the solvent was removed under vacuum. All compounds gave an oily solid, which were passed through a short silicagel column (80% ether, 20% petrol ether). The resulting fractions were dried from excess solvent under a stream of argon to yield crystals.
4-Chloro-5-hydroxy-1-methyl-5-phenyl-1,5-dihydro- pyrrol-2-one 1
Yield = 75 %; mp: 146-148oC; MS (APC1(+)): 193/195 (M+1), 224/226 (M+) m/z; *H NMR (DMSO-d6)) 250 MHz: 7.29-7.48 (m, 5H), 6.49 (s, 1H), 2.08 (s, 3H) 13C NMR (CDCl3) 168.1, 156.4, 134.1, 129.4, 128.9, 126.2, 121.3, 92.6, 24.5 ppm. IR (KBr-disc): 3224, 3110, 2952, 2820, 2617, 2375, 2339, 1975, 1697, 1605, 1453, 1438, 1258, 1207, 1065, 992, 856, 764, 704 cm-1.
4-Chloro-5-(4-chloro-phenyl)-5-hydroxy-1-methyl- 1.5-dihydro-pyrrol-2-one 2
Yield = 66 %; mp: 179-181oC; MS (APC1(+)): 227/229/231 (M+1), 258/260/262 (M+) m/z; *H NMR (CDCl3) 250 MHz: 7.317.42 (m, 4H), 6.06 (s, 1H), 4.56-4.71 (bs, 1H), 2.60 (s, 3H) 13C NMR (CDCl3) 167.8, 156.0, 135.5, 132.8, 129.1, 127.8, 121.6, 92.2, 24.4 ppm. IR (KBr-disc) 3429, 3102, 2970, 2932, 2857, 1677, 1611, 1494, 1475, 1431, 1202, 1151, 1091, 988, 928, 811, 692 cm-1.
4-Chloro-5-hydroxy-1-isopropyl-5-phenyl-1,5- dihydro-pyrrol-2-one 3
Yield = 79 %; mp: 163-165oC; MS (APC1(+)): 193/195 (M+1), 252/254 (M+) m/z; 1H NMR (CDCl3) 250 MHz: 7.40-7.51 (m, 5H), 6.14 (s, 1H), 3.81(bs, 1H), 3.42 (m, 1H), 1.33& 1.21 (m, 6H) 13C NMR (CDCl3) 167.5, 155.0, 135.0, 129.1, 128.5, 126.4, 122.4, 93.4, 45.6, 21.1, 20.0 ppm. IR (KBr-disc) 3227, 2990, 2940, 2365, 2350, 1956, 1693, 1615, 1456, 1428, 1247, 1131, 1072, 1009, 934, 847, 747, 697 cm-1.
4-Chloro-5-(4-chloro-phenyl)-5-hydroxy-1-isopropyl- 1.5-dihydro-pyrrol-2-one 4
Yield =69 %; mp: 127-130oC; MS (APC1(+)): 286/288/290 (M+) m/z; *H NMR (CDCl3) 250 MHz: 7.31 (m, 4H), 6.06 (s, 1H), 3.33 (m, 1H), 1.25 & 1.10 (m, 6H). 13C NMR (CDCl3) 167.1, 154.0, 136.7, 133.4, 128.9, 128.0, 123.2, 92.9, 45.6, 20.1, 21.3 ppm. IR (KBr-disc) 3272, 2978, 2927, 1691, 1614, 1496, 1429, 1384, 1352, 1249, 1096, 1012, 936, 846, 801, 683 cm-1.
4-Chloro-1-cyclopropyl-5-hydroxy-5-phenyl-1,5- dihydro-pyrrol-2-one 5
Yield = 83 %; mp: 177-179oC; MS (APCI(+)): 193/195 (M+1), 250/252 (M+) m/z;*H NMR (CDCl3) 250 MHz: δ = 7.41 (m, 5H), 6.09 (s, 1H), 3.50 (m, 1H), 2.18 (m, 1H), 0.95 & 0.38 (m, 4H); 13C NMR (CDCl3) 167.4, 154.8, 135.2, 129.2, 128.8, 126.1, 122.2, 93.5, 22.6, 3.8, 5.1 ppm. IR (KBr-disc) 3416, 3260, 3105, 3011, 2363, 2338, 1671, 1602, 1490, 1450, 1409, 1369, 1256, 1144, 1032, 939, 833, 752, 702 cm-1.
4-Chloro-5-(4-chloro-phenyl)-1-cyclopropyl-5- hydroxy-1,5-dihydro-pyrrol -2-one 6
Yield = 72 %; mp: 169-171oC; MS (APCI(+)): 284/286/288 (M+) m/z; *H NMR (CDCl3) 250 MHz: 7.22 (m, 4H), 5.97 (s, 1H), 3.98(bs, 1H), 1.76 (m, 1H), 0.24-0.99 (m, 4H); 13C NMR (CDCl3) 165.8, 155.4, 144.2, 133.7, 129.0, 127.7, 122.2, 91.7, 22.6 , 3.7, 5.2 ppm. IR (KBr-disc) 3433, 3220, 3019, 2935, 2858, 1700, 1675, 1497, 1412, 1251, 1209, 1144, 1089, 1015, 940, 844, 802, 679 cm-1.
4-Chloro-5-hydroxy-1-isobutyl-5-phenyl-1,5-dihydro- pyrrol-2-one 7
Yield = 85 %; mp: 167-169oC; MS (APCI(+)): 266/268 (M+) m/z; *H NMR (CDCl3) 250 MHz: 7.38-7.51 (m, 5H), 6.24 (s, 1H), 4.79 (bs, 1H), 3.23 & 2.18 (m, 2H), 1.71 (m, 1H), 0.76 (m, 6H) 13C NMR (CDCl3) 168.5, 155.7, 137.1, 129.2, 128.7, 126.2, 121.7, 93.1, 47.6, 27.5, 20.4 ppm. IR (KBr-disc) 3237, 3114, 2965, 2926, 2881, 2374, 2343, 1675, 1614, 1460, 1416, 1299, 1251, 1202, 1150, 1072, 1027, 878, 758, 696 cm’-1.
4-Chloro-5-(4-chloro-phenyl)-5-hydroxy-1-isobutyl- 1,5-dihydro-pyrrol-2-one 8
Yield = 66%; mp: 155-158oC; MS (APCI(+)): 300/302/304 (M+) m/z; *H NMR (CDCl3) 250 MHz: 7.30 (m, 4H), 6.19 (s, 1H), 3.13 (m, 1H), 2.49 (m, 1H), 1.69 (m, 1H), 0.69 (t, J = 4.5 Hz, 6H) 13C NMR (CDCl3) 163.3, 156.3, 139.4, 134.8, 129.1, 127.7, 122.3, 95.0, 47.6, 27.6, 20.4 ppm. IR (KBr-disc) 3426, 3252, 2964, 2850, 1684, 1406, 1209, 1095, 817, 743, 703 cm--1.
4-Chloro-1-cyclopentyl-5-hydroxy-5-phenyl-1,5- dihydro-pyrrol-2-one 10
Yield = 81 %; mp: 180-182oC; MS (APCI(+)): 278/280 (M+) m/z; 1H NMR (CDCl3) 250 MHz: δ = 7.51(m, 5H), 6.08 (s, 1H), 4.87 (bs, 1H), 3.59 (m, 1H), 1.99 (m, 2H), 1.81 (m, 4H), 1.46 (m, 4H); 13C NMR (CDCl3) 167.2, 155.0, 135.2, 129.1, 128.6, 126.5,
122.2, 93.3, 54.3, 30.0, 28.8, 24.5, 24.4 ppm. IR (KBr-disc) 3220, 2961, 2877, 2373, 2341, 1684, 1613, 1448, 1426, 1248, 1199, 1141, 1070, 934, 850, 750, 701 cm--1.
4-Chloro-5-(4-chloro-phenyl)-1-cyclopentyl-5- hydroxy-1,5-dihydro-pyrrol-2-one 11
Yield = 73 %; mp: 157-159oC; MS (APCI(+)): 312/314/316 (M+) m/z; 1H NMR (CDCl3) 250 MHz: δ = 7.42 (m, 4H), 6.03 (s, 1H), 4.99 (bs, 1H), 3.51-3.62 (m, 1H), 1.97-2.19 (m, 2H), 1.681.93 (m, 8H); 13C NMR (CDCl3) 167.1, 154.8, 135.2, 133.9, 128.9, 128.0, 122.3, 93.0, 54.3, 30.0, 28.9, 24.5 ppm. IR (KBr-disc) 3407, 3276, 2968, 2922, 2883, 2379, 2339, 1691, 1491, 1429, 1367, 1249, 1203, 1092, 1013, 932, 843, 787, 709 cm-1.
4-Chloro-1-hexyl-5-hydroxy-5-phenyl-1,5-dihydro- pyrrol-2-one 12
Yield = 51 %; mp: 173-175oC; MS (APCI(+)):294/296 (M+) m/z; 1H NMR (CDCl3) 250 MHz: 7.40 (m, 5H), 6.15 (s, 1H), 4.76 (bs, 1H), 3.28 (m, 1H), 2.91 (m, 1H), 1.09-1.59 (m, 8H), 0.78-0.92 (t, J = 7.1 Hz, 3H) 13C NMR (CDCl3) 168.0, 155.6, 134.9, 129.2, 128.7, 126.2, 121.8, 93.0, 40.2, 31.3, 28.7, 26.8, 22.5, 14.0 ppm. IR (KBr-disc) 3245, 2930, 2865, 1689, 1658, 1494, 1453, 1412, 1365, 1321, 1150, 1069, 927, 753, 696 cm'-1.
4-Chloro-5-(4-chloro-phenyl)-1-hexyl-5-hydroxy-1,5- dihydro-pyrrol-2-one 13
Yield = 49 %; mp: 169-172oC; MS (APCI(+)): 328/330/332 (M+) m/z; 1H NMR (CDCl3) 250 MHz: 7.31(m, 4H), 6.15 (s, 1H), 3.24 (m, 1H), 2.67 (m, 1H), 1.04-1.69 (m, 8H), 0.74 (t, J = 6.3 Hz, 3H) 13C NMR (CDCl3) 165.8, 155.7, 140.8, 136.9, 129.1, 127.8,
91.6, 40.3, 30.8, 29.1, 26.8, 22.6, 15.2 ppm. IR (KBr-disc) 3446, 2935, 2863, 1698, 1413, 1252, 1200, 1138, 1092, 1013, 938, 846, 814, 702 cm-1.
4-Chloro-1-cyclohexyl-5-hydroxy-5-phenyl-1,5- dihydro-pyrrol-2-one 14
Yield = 57 %; mp: 170-172oC; MS (APCI(+)): 292/294 (M+) m/z; 1H NMR (CDCl3) 250 MHz: 7.26-7.61 (m, 5H), 6.08 (s, 1H), 3.77 (bs, 1H), 2.88 (m, 1H), 1.21-2.07 (m, 10H); 13C NMR (CDCl3) 163.9, 153.9, 135.0, 129.25, 128.9, 126.4, 122.9 , 96.0, 53.6, 32.8,
31.1, 29.8, 26.2 , 24.2 ppm. IR (KBr-disc) 3440, 2924, 2858, 2355, 2344, 1641, 1449, 1367, 1250, 1138, 1016, 996,742, 695 cm4
4-Chloro-1-(phenyl)-5-hydroxy-5-phenyl-1,5-dihydro- pyrrol-2-one 15
Yield = 48 %; mp: 168-171oC; MS (APCI(+)): 314/316 (M+) m/z; 1H NMR (CDCl3) 250 MHz: δ = 7.46 (m, 5H), 7.34 (m, 5H), 6.38 (s, 1H), 3.68 (bs, 1H); 13C NMR (CDCl3) 168.9, 159.7, 136.9, 135.1, 132.4, 129.9, 129.0, 126.9, 123.0, 122.3, 122.2, 93.5; IR (KBr-disc) 3517, 3357, 3114, 2840, 2674, 2361, 2342, 1678, 1607, 1464, 1412, 1361, 1208, 1138, 1071, 988, 755, 700 cm-1.
Molecular Modeling
For target preparation the protein structures, pdb identifier 1HZN for the CCKj and 1L4T for the CCK2-gastrin receptor were downloaded from the protein data bank and docking was performed using Auto dock Vina and Hex. After several docking trials for the CCKj / CCK2 receptor the results were analysed and visualized using Chimera and Designer studio 4.5. After visual inspection the results were presented to rationalize drug ligand interactions with the each CCK receptor subtype.
Radioligand Cholecystokinin Binding Assay
CCK2 and CCKj receptor binding assays were performed, by using guinea pig cerebral cortex or rat pancreas. Male guinea pig brain tissues were prepared according to the modified method described by Saita et al. [28]. Pancreatic membranes were prepared as described by Charpentier et al. [29]. Tissues were homogenized in ice cold sucrose (0.32 M, 25 ml) for 15 strokes at 500 rpm and centrifuged at 13000 rpm for 10 minutes. The supernatant was re-centrifuged at 13000 rpm for 20 minutes. The resulting pellet was re-dispersed to the required volume of buffer at 500 rpm and stored in aliquots at 700C. Binding was achieved using radio ligand 1251-Bolton-Hunter labeled CCK, NEN at 25 pM. The samples were incubated with membranes (0.1 mg/ ml) in 20 mM Hepes, 1 mM EGTA, 5 mM MgCl2, and 150 mM NaCl, at pH 6.5 for 2 hrs at RT and then centrifuged at 11000 rpm for 5 minutes. The membrane pellets were washed twice with water and the bound radioactivity was measured in a Packard Cobra Auto-gamma counter (B5005). Binding assays were carried out with L-363, 260 as control.
Isolated Tissue Preparations
Male Sprague Dawley rats, weighing 200-250g were used and all animal care and experimental protocols adhered to the relevant laws and guidelines of the institution. The animals were housed under standard conditions of temperature (25°C) with unrestricted access to food and water. The animals were sacrificed using cervical dislocation without anaesthesia. From the abdomen of the animals, the duodenum was carefully excised and washed with physiological solution. The mesentery of the tissue was removed and the lumen was gently flushed with Tyrode's solution to clear luminal contents. The prepared isolated tissue was rapidly incubated in Tyrode's solution maintained at 32oC and gassed with 95% O2 / 5% CO2. Tyrode's solution was freshly prepared daily (g/l): NaCl, 8.0; KCl, 0.2; CaCl2, 0.2; MgSO4, 0.1; NaH2PO4, 0.05; NaHCO3, 1.0; Glucose, 1.0. The main equipment used was the Radnoti single unit tissue bath system with a chamber capacity of 35 ml. Bath aeration with carbogen (O2 95%, CO2 5%) was maintained at a constant temperature (32°C). The force in grams was measured with an isometric transducer linked to a power lab data acquisition system.
General Procedure
From the isolated tissue preparation, strips of appropriate length were mounted vertically in organ bath containing Tyrode's solution, under a tension of 1g and allowed to equilibrate for 30 minutes. Agonists, such as CCK8S were directly applied in the bath and antagonists were pre-incubated for 10 min. Stock solutions of all test compounds including the standard were prepared in DMSO.
Cholecystokinin CCK8 Preparations
CCK8S was dissolved in distilled water to prepare a stock solution of 500 μM solution, from which cumulative additions of increasing concentrations (0.1 nM, 1 nM, 5 nM, 10 nM, 20 nM, 30 nM, and 40 nM) were tested to plot a dose response curve. Test molecules and lorglumide were added to the organ bath 10 minutes before exposure to the next CCK8S serial concentrations.
Animal Studies
Experiments were conducted in male standard 1RC mice obtained from the animal house, Faculty of Medicine, Khon Kaen University. Each experimental group consisted of 6 animals and the treatment procedures were approved by the ethical committee, Faculty of Medicine, Khon Kaen University (BEA030699). Mice were intraperitoneal injected with either test compound dissolved in 5% DMSO at the volume not more than 0.2 ml/animal. At 30 min after treatment, animals were tested as described in the following sections.
Nociception Tests
The Tail Immersion Test: The thermal response latency was measured by the tail immersion test. The animals were placed into individual restraining cages leaving the tail hanging freely. The tail was immersed into water at 50oC. The response time, at which the animal reacted by withdrawing its tail from water, was recorded and the cut-off time was 10 sec in order to avoid tissue damage. The base line withdrawal thresholds (BT) were recorded prior to the first injection. Test thresholds (TT) were measured 60 min after the second injection.
The test thresholds were expressed as a percentage of Maximal Possible Effect (% MPE) using the equation:
%MPE = {(TT - BT) / (45 - BT)}X100
DMSO (5 %), pyrrolone (in 5 % DMSO) was intraperitoneally injected and morphine was administered subcutaneously.
Anxiolytic Test
The Elevated Plus-Maze: The wooden elevated plus-maze consisted of two open arms (30 x 10 cm) without any walls, two enclosed arms of the same size with 5-cm high side walls and end wall, and the central arena (10 x 10 cm) interconnecting all the arms. The maze was elevated approximately 30 cm from the floor. At the beginning of the experiment the mouse was placed in the central arena facing one of the enclosed arms. During a 5-min interval, the time animals spent in the open arms of the plus-maze was recorded. The mouse was considered to be in the open part when it had clearly crossed the line between the central arena and the open arm with its four legs.
Statistical Methods
The data were expressed as mean + SD and one-way analysis of variance and supplementary Tukey test for pairwise comparison were tested to determine for any significant difference at p< 0.05.
Results and Discussions
Chemistry
5-arylated dichloro-2(5H)-furanones A and B were synthesised from mucochloric acid (Figure 2), which is commercially available from furfural under oxidising conditions with hydrochloric acid. Theses intermediates were evaluated previously as anticancer agents [30]. Mucochloric acid was reacted with benzene as reagent and solvent at RT under the development of hydrogen chloride gas. Depending on the scale of the reaction cooling with ice was required. For chlorobenzene / benzene the powdered or most preferred granulated aluminium chloride served as the best catalyst and during work up with hydrochloric acid on ice the inorganic salts were easily removed from the organic phase. In terms of scope of the reaction arylated 2(5H)-furanones, containing nitro- groups or trifluoro-methyl groups could not be prepared. For the small scale synthesis aluminium chloride worked well as Lewis acid. However, during scale up aluminium chloride was replaced by trifluoroborane in THF as the exothermic reaction become problematic on a kg scale.
Subsequent reaction of the 5-arylated 3,4-dichloro-2(5H)- furanones A and B (Stage 1 intermediate) in diethylether with alkyl- and aryl alkyl amines furnished N-alkylated hydroxyl- pyrrolones 1-15 ( Stage 2 products ) in high yields under mild conditions. The general synthetic sequence is outlined in Figure 2. Overall, the desired N-alkylated unsubstituted 5-phenyl pyrrolones 1-15 were obtained in only a 2 stage process as white crystalline material. The molecule is not present in the ring opened keto form and fullyoCcurred in the 5-membered ring form, as a hydroxy-pyrrolone. The 5-arylated 2(5H)-furanones reacted selectively in the ester position and no reaction in the 4-position was observed here. Previously the IPSO substitution (4-position) was described for pseudo- esters, and here in Scheme 1, a ring-opening ring-closure mechanism is proposed for the formation of hydroxy-pyrrolones [31].
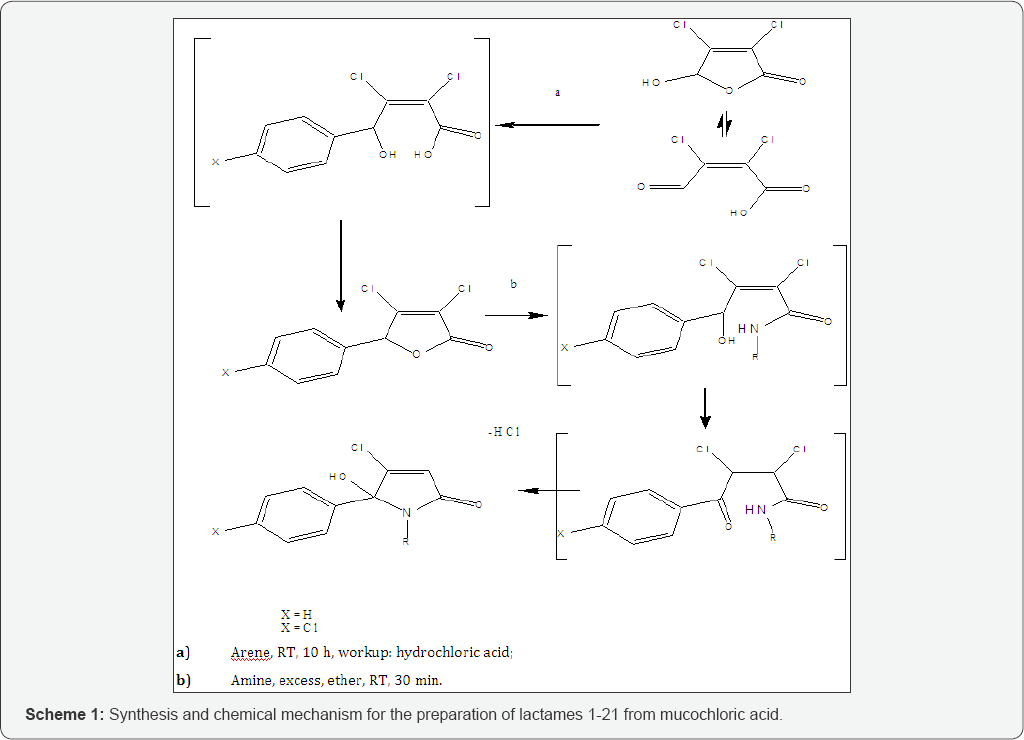
Thus, the first step in the reaction sequence of the dichlorinated 2(5H)-furanone is the ring opening and amide formation from the corresponding lactone. Subsequently, the keto form of the acyclic amide was in situ converted into a lactame under the elimination of hydrogen chloride (middle, Figure 2). The analysis of lactame 7 by chiral HPLC showed a 50:50 racemic mixture of both enantiomers in solution in methanol.
SAR Optimisation
The first step was to screen for potent binding affinity and to identify a CCK1 or CCK2-selective ligand for subsequent in vitro and in vivo evaluation. Using radiolabelled iodinated cholecystokinin, inhibition of binding was determined for all test molecules and the lC50 are outlined in Table 1. Lorglumide served as CCK1 standard and L-365,260 was used as CCK2 standard.Hydroxy-pyrrolones containing an N-methyl group, present in lactame 1-2, showed no binding affinity and an increase in the length of the N- side chain resulted in micromolar binding affinity for the iso-propyl group, as seen in lactame 3 and 4. Ring closure on the N-substituent, from isopropyl to cyclo-propyl resulted in a loss of activity (lactame 5, 6) and the ring enlargement from a 3- membered to a 5- membered ring system (entry 10-11) resulted in no significant increase of binding affinity.
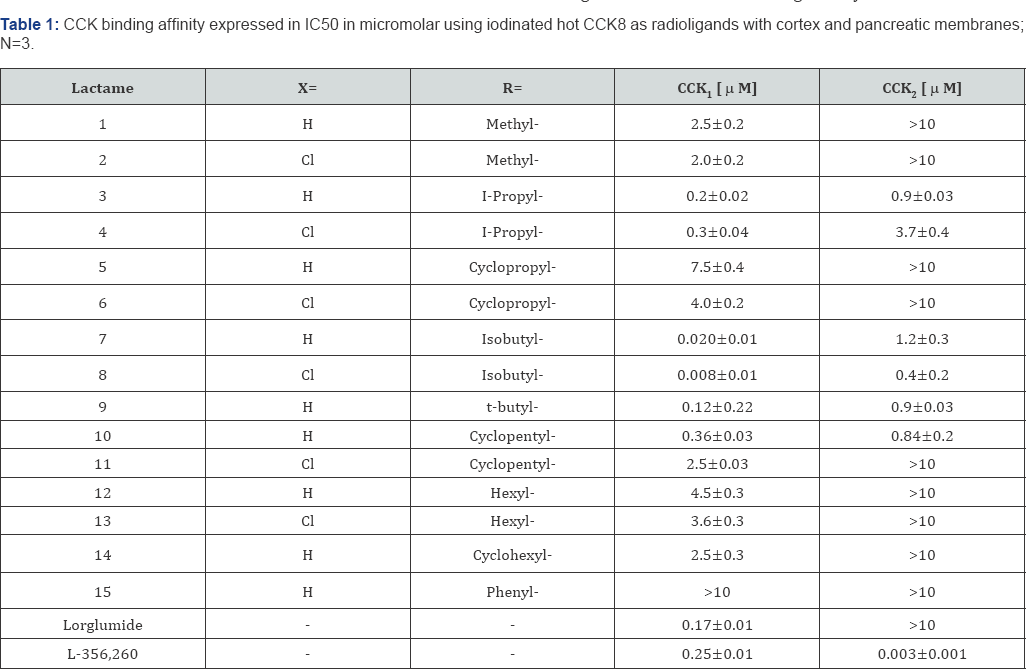
The change from N-propyl into the N-butyl group resulted in a manifold increase of activity and the best substituent on the central nitrogen atom was found iso-butyl, as seen for derivative 7. The introduction of a halogen atom into the para- position of the phenyl group resulted in an increase of binding affinity, possibly due to enhanced lipophilicity. The iso-butyl group on the N-atom produced the best overall ligand, with CCKj selectivity and the 1C50 was reduced significantly for t-butyl derivative 9. The n-pentyl analogue was formed in very low yields and the n-hexyl lactames 12, 13 clearly lost binding affinity and the same was observed for the cyclohexyl derivative 14. Lactames, such as pyrrolone 15, containing an aromatic ring, directly connected to the N-position, showed a micromolar activity > 10 μ M. Overall, the introduction of alkyl groups, most preferred an isobutyl- group, provided a CCKj selective antagonist PNB-081, which was the selected development candidate. A fluorinated analogue had potent anticancer properties via the CCKc receptor (Figure 2) [32].
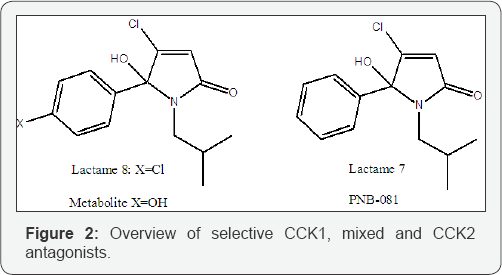
Lactame 8 (pA2 = 8.1) has a nominally higher binding affinity compared with lactame 7 (Lactame 7 = PNB-081, pA2 = 7.7) but they were found identical in vivo. In addition PNB-081 has a higher melting point (higher chemical stability) and better physical properties (crystal properties). 1t can be produced during scale up with an increasing chemical yield and excellent (>99.7 %) purity, required by cGMP synthesis. Therefore, PNB- 081 chosen for preclinical development. Most importantly, in vivo it may be metabolized into its para hydroxy-analogue, in line with its pharmacological profile.
Molecular Modelling
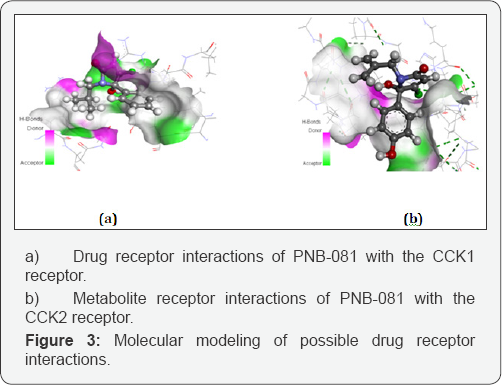
Molecular modelling studies were performed for PNB-081 with the CCKj receptor (Figure 3). The isobutyl group of the ligand interacted with a hydrophobic cave of the receptor, centred at Ala-14. The carbonyl group in the 2- position bond via hydrogen binding towards the CCK receptor with Arg-9 and the N- atom of the lactame interacted with Glu-17. The 5-hydroxy- group of the ligand displayed interactions with of Asn-6, while the phenyl group has no interaction with tryptophan or phenylalanine. Pi-alkyl interactions only may explain the small increase in binding affinity of the chlorinated analogue of PNB-081, based on interaction with Leu-29 and Ile-28. If the para phenyl position is not blocked by the chorine atom, most interestingly, the proposed metabolite can interact with the CCK2 receptor. It is supposed that PNB-081, is hydroxylated in the para phenyl position by P450 and this metabolite may interact with the CCK2 receptor via His 122 interaction, outlined in Figure 3. The role of metabolites is currently under investigations. Most interestingly hydroxylation may also enhance CCKt affinity due to interactions with Arg 9 (Figure 3). Gaining dual CCK-gastrin antagonistic activity was found beneficial in analgesia potentiation as well as for anxiolytics [33,34].
Pharmacology
In Vitro Experiments Using Isolated Tissue Preparations: Initially CCK4 was used, an agent, which trigged panic attacks in patients, but in vitro CCK4 has a low solubility and low potency in the micro-molar range [35]. The best full CCK agonist in vitro and in humans is CCK8s [36]. Cholecystokinin, CCK8s, induced contractions of the guinea pig gall bladder and this tissue based assay was adapted to the rat duodenum preparation [37]. CCK-8s induced dose dependently contractions of the rat duodenum over a wide concentration range. These contractions were reduced dose dependently for PNB-081, which is outlined in Figure 4.
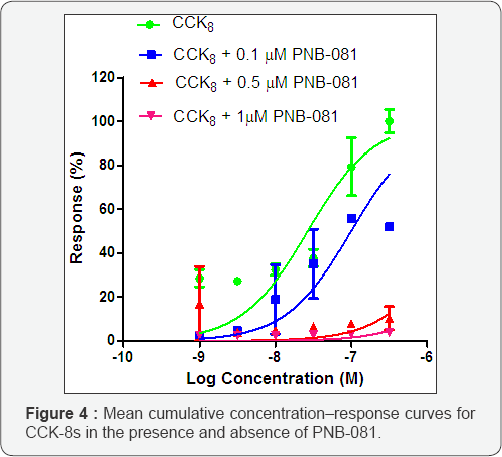
Increased concentrations of the antagonist, PNB-081 were added to the bath cumulatively and a shift of the curve to the right was observed. PNB-081 was acting as non-competitive antagonist [38]. The problem with the gall bladder based assay is the limit of the tissue and the rat duodenum represented an excellent alternative with a good expression of the CCKj receptor In conclusion, the CCK antagonising properties of PNB-081 were clearly established using selective tissues and selective ligands. Under consideration of failed clinical trials for panic, positive pain results, our attention turned to a systematic in vivo evaluation of the agent as an adjunct to opiates [39].
In Vivo Evaluation: In order to evaluate the pain managing properties of PNB-081, the tail immersion assay were used in rodents. CCK antagonists potentiated the analgesia of opiates and usually (Lattmann, 2016) have no analgesic effect on their own.

Potentiation of Opiate Analgesia: It was now focussed on the potentiation of morphine analgesia for our selected cholecystokinin antagonist PNB-081. The analgesic effect of morphine served as baseline and a linear correlation of the response time in s against the log dose of morphine from 2 to 16 mg/kg was obtained in the tail immersion tail immersiontest in mice (Figure 5). An IP dose of 0.5 and 1.5 mg/kg of PNB-081 potentiated the low morphine dose by factor 3. The 2 mg/kg dose of morphine was shifted in presence of the CCK antagonist to an equivalent dose of 16 mg/kg morphine. Previously 2 different chemical classes, the anilino- benzodiazepines Lattmann 2006 and pyrazols Lattmann 2005 showed pain potentiation in the same standard assays.
Opiate Tolerance: Opiates and endorphines, so small organic molecules as well as peptides act as opiate agonist and produce analgesia. This analgesic effect is reversed by cholecystokinin, not gastrin. The established model of tolerance is that, opiates induce the formation of cholecystokinin receptors and then cholecystokinin in form of CCK8s, which is the main circulating form, neutralises opiate analgesia. A CCK antagonist does reverse this by blocking the newly formed CCK receptors [40].
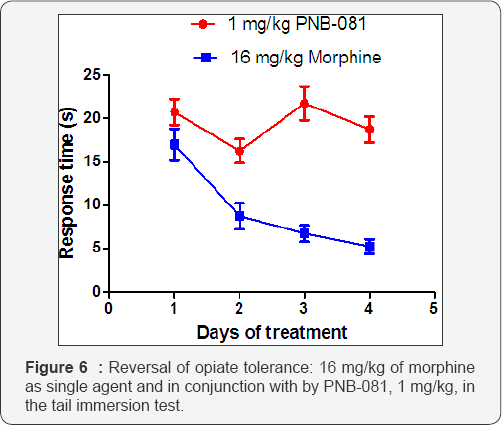
Experimentally, in line with the proposed model, a 16 mg / kg dose of morphine, exhibited the anticipated analgesic effect and this analgesia faded out within days based on bydaily sc administration of morphine (Figure 6). In presence of 1 mg PNB- 081 administed intraperitonally, no tolerance was observed and a continuous analgesic effect was observed over the duration of 4 days. This is clear confirmation, that opiates and cholecystokinin are supposed to have opposing effects. Prolonged exposure to opiates induced the expression of CCK receptors and these CCK receptors were blocked by the CCK antagonist PNB-081. PNB- 081 may be hydroxylated in vivo in mice gaining CCK2 selectivity [41]. Thus, a dual acting CCK antagonist may be produced, alleviating best the anti-nociceptive effects of morphine.
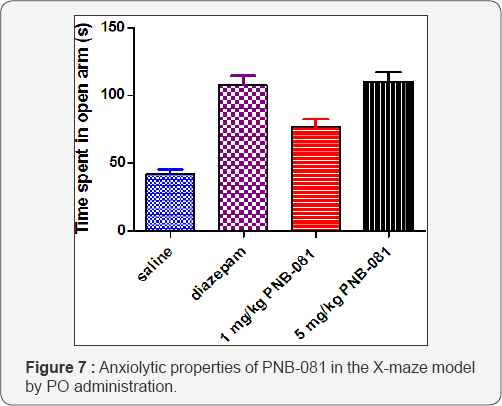
To complete the CNS evaluation of PNB-081, a standard anxiolytic assay, the X-maze test was applied using mice (Figure 7). Mice in general dislike open areas and the anxiolytic diazepam (4 mg/kg) increased the time significantly they spent in open arms. For PNB-081 an anxiolytic effect was observed for the 1 mg/kg dose and this increase further for the 5 mg/kg dose by PO administration. CCK4 induced panic in humans and generally CCK2 antagonists are associated with anxiolytic properties. Our finding are supported by other selected CCK1 antagonists and the detection of CCK1 receptors in wide key areas of the brain [42,43]. Additionally, an estimate of the oral bioavailability (2025%) was derived from this assay. As part of the preclinical development, the pharmacokinetics of PNB-081 was fully analysed. In summary PNB-081 showed a good half-life in dogs and rats. Protein binding was determined of 82.4% in human plasma and very high membrane permeability was determined by using the Caco-2 monolayer assay for both molecules. PNB- 081 has an oral bioavailability of 2 5% in rats and PNB-081 entered preclinical toxicology.
Conclusion
The target molecule PNB-081 was synthesised in only 2 steps from one readily available starting materials and will potentially deliver affordable therapeutic agents for long term pain management. A first in class analgesic CCK antagonist was developed under the consideration of membrane penetration, half-life and bioavailability. Overall the cholecystokinin antagonist PNB-081 has shown the expected CNS profile with opiate potentiation and with anxiolytic properties. Pain as well as CNS trials are hard and unpredictable with high placebo response and therefore, for PNB-081 pancreatitis may pose a possible route through regulatory approval. Potentiation of opiate analgesia, as well as the reversal of opiate tolerance is clear clinical features of the CCK1 antagonist PNB-081, which may help to alleviate the current opiate crisis in industrialised countries.
Acknowledgement
The experimental work was partly supported by PNB Vesper Life Sciences. The animal studies were performed in compliance with relevant laws and institutional guidelines.
References
- Maton P, Sutliff V, Jensen R (1985) Carbobenzoxy amino acids: Structural requirements for cholecystokinin receptor antagonist activity. Am J Physiol 248: 479-484.
- Herranz R (200S) Cholecystokinin Antagonists: Pharmacological and Therapeutic Potential. Med Res Rev 2S(S): 559-605.
- Mawe GM (1991) The role of cholecystokinin in ganglionic transmission in the guinea-pig gall-bladder. J Physiol 4S9: 89-102.
- Jorpes E, Mutt V (1966) Cholecystokinin and Pancreozymin, one single Hormone? Acta Physiol Scand 66(1-2): 196-202.
- Mc Donald IM (2001) CCK2 receptor antagonists. Exp Opin Ther Patents 11: 44S.
- Bock MG, Di Pardo RM, Mellin EC (1994) Second-generation benzodiazepine CCK-B antagonists. Development of subnanomolar analogues with selectivity and water solubility. J Med Chem S7(6): 722-724.
- Lattmann E, Arayarat P (2003) From CNS-drugs to anti-neoplastic agents: Cholecystokinin (CCK) antagonists as modern anti-cancer agents. KKU Science J S1: 178.
- Dourish CT (1990) Cholecystokinin and anxiety. Trends Pharmacol Sci 11(7): 271-27S.
- Rasmussen K, Czachura, JF, Stockton, ME (199S) Electrophysiological effects of diphenylpyrazolidinone cholecystokinin-B and cholecystokinin-A antagonists on midbrain dopamine neurons. J Pharmacol Exp Ther 264(1): 480-488.
- Dourish CT, Rycroft W, Iversen SD (1989) Postponement of satiety by blockade of brain cholecystokinin-B receptors. Science 245: 1509- 1311.
- Bradwejn J, Koszycki D, Meterissian G (1990) Cholecystokinin tetrapeptide induces panic attacks in patients with panic disorder. Can J Psychiaty SS(1): 8S-8S.
- Hughes J, Boden P, Costallt B (1990) Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc Natl Acad Sci USA 87(17): 6728-6732.
- Trivedi K, Bharat J (1994) Cholecystokinin receptor antagonists: Current status. Curr Med Chem 1: S1S.
- Chang R, Lotti V, Monaghan R (1985) A potent nonpeptide cholecystokinin antagonist selective for peripheral tissues isolated from Aspergillus alliaceus. Science 2S0: 177-179.
- Lattmann E, Billington DC, Poyner DR (2001) Synthesis and evaluation of Asperlicin analogues as non-peptidal Cholecystokinin-antagonists. Drug Des Discov 17(S): 219-2S0.
- Evans BE, Rittle KE, Bock MG (1988) Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin-A antagonists. J Med Chem S1(12): 22SS-2246.
- Hahne W, Jensen R, Lemp G (1981) Proglumide and benzotript: Members of a different class of cholecystokinin receptor antagonists. Proc Natl Acad Sci USA 78(10): 6304-6308.
- Makovec F, Chisté R, Bani M (1985) New glutaramic acid derivatives with potent competitive and specific cholecystokinin-antagonistic activity. Arzneimittelforschung SS(7): 1048-10S1.
- Noble F, Wank SA, Crawley JN (1999) International Union of Pharmacology XXI. Structure, Distribution, and Functions of Cholecystokinin Receptors. Pharmacol Rev S1(4): 74S-781.
- Woodruff GN, Hughes J (1991) Cholecystokinin antagonists. Annu Rev Pharmacol Toxicol S1: 469.
- Lattmann E, Sattayasai J, Schwalbe CH (2016) Analgesic Effects of S-Alkyloxy-4-amino-2(SH)-furanones as Cholecystokinin-2 antagonists. Arch Pharm (Weinheim) S49(6): 4S6-46S.
- Yekkirala AS, Roberson DP, Bean BP (2017) Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 16(8): 545-564.
- Dourish C, Neill M, Coughlan J (1990) The selective CCK-B receptor antagonist L-S6S,260 enhances morphine analgesia and prevents morphine tolerance in the rat. Eur J Pharmacol 176(1): SS-44.
- Simpson KH, Serpell M, McCubbins TD (2002) A multi-dose study: Management of neuropathic pain in patients using a CCK antagonist devazepide (DEVACADE®) as an adjunct to strong opioids. In World Congress of 10th IASP, San Diego, CA, USA.
- Lattmann E, Russell ST, Schwalbe CH (2016) Cholecystokinin-1 receptor antagonists: S-hydroxy-S-aryl-pyrrol-2-ones as anticancer agent. Med Chem Commun 7(6): 11S8-114S.
- Lattmann E, Sattayasai J, Narayanan R (2017) Cholecystokinin-2/gastrin antagonists: 5-hydroxy-5-aryl-pyrrol-2-ones as anti-inflammatory analgesics for the treatment of inflammatory bowel disease. Med Chem Commun 8(S): 680-68S.
- Baber S, Dourish T, Hill R (1989) The role of CCK caerulein and CCK antagonists in nociception. Pain S9(S): S07-S28.
- Saita Y, Yazawa H, Honma Y, Nishida A, Miyata K, et al. (1994) Characterization of YM022: its CCKB/gastrin receptor binding profile and antagonism to CCK-8-induced Ca2+ mobilization. Eur J Pharmacol 269(2): 249-281.
- Charpentier B, Pelaprat D, Durieux C, Dor A, Reibaud M, et al. (1988) Cyclic cholecystokinin analogues with high selectivity for central receptors. Proc Natl Acad Sci USA 85(6):1968-1973.
- Lattmann E, Ayuko WO, Kinchinaton D (200S) Synthesis and evaluation of S-arylated 2(SH)-furanones and 2-arylated pyridazin-S(2H)-ones as anticancer agents. J Pharm Pharm SS(9): 12S9-126S.
- Lattmann E, Sattayasai N, Schwalbe CS (2006) Novel anti-bacterials against MRSA: synthesis of focussed combinatorial libraries of trisubstituted 2(SH)-furanones. Curr Drug Discov Technol 3(2):125-134.
- Ponnusamy S, Lattmann E, Lattmann P (2016) Novel, isoform-selective, cholecystokinin A receptor antagonist inhibits colon and pancreatic cancers in preclinical models through novel mechanism of action. Oncol Rep SS(4): 2097-2106.
- Lattmann E, Boonprakob Y, Sattayasai J (2008) Part 2. Long term in vivo/ in vitro evaluation of the Cholecystokinin antagonists: N-(5-methyl-3- oxo-1,2-diphenyl-2,3-dihydro-1H-pyrazol-4-yl)-N'-phenylurea MPP and carboxamide MPM. Drug Discov Ther 2(6): S44-SS2.
- Revel L, Mennuni L, Garofalo P (1998) CR 2945: A novel CCKB receptor antagonist with anxiolytic like activity. Behav Pharmacol 9(3): 183194.
- Bradwejn J, Koszycki D, Couetoux du Tertre A (1994) The panicogenic effects of cholecystokinin-tetrapeptide are antagonized by L-S6S,260, a central cholecystokinin receptor antagonist, in patients with panic disorder. Arch Gen Psychiat S1(6): 486-49S.
- Amato M, Stamford I, Bennett A (1990) The effects of cholecystokinin octa-peptide on human isolated alimentary muscle. Br J Pharmacol 100(1): 126-1S0.
- Bishop L, Gerskowitch V, Hull R (1992) Combined dose-ratio analysis of cholecystokinin receptor antagonists, devazepide, lorglumide and loxi- glumide in the guinea-pig gall bladder. Br J Pharmacol 106(1): 61-66.
- Kenakin T, Jenkinson S, Watson C (2006) Determining the Potency and Molecular Mechanism of Action of Insurmountable Antagonists. J Pharmacol Exp Ther 319(2): 710-723.
- Sramek JJ, Kramer MS, Reines SA (1994) Pilot study of a CCKB antagonist in patients with panic disorder: Preliminary findings. Anxiety 1(3): 141-143.
- Wiesenfeld-Hallin Z, Xu XJ (1996) The role of cholecystokinin in nociception, neuropathic pain and opiate tolerance. Regul Pept 65(1): 23-28.
- Zhou Y, Sun Y, Zhang Z (1993) Increased release of immunoreactive cholecystokinin octapeptide by morphine and potentiation of mu- opioid analgesia by CCKB receptor antagonist L-365,260 in rat spinal cord. Eur J Pharmacol 234(2-3): 147-154.
- Ballaz S, Barber A, Fortuno A (1997) Pharmacological evaluation of IQM-95,333, a highly selective CCKA receptor antagonist with anxiolytic-like activity in animal models. Br J Pharmacol 121(4): 759767.
- Zarbin M, Innis R, Wamsley J (1983) Autoradiographic Localization of Cholecystokinin Receptors in Rodent Brain. J Neurosci 3(4): 877-906.






























