Method Development and Validation for the Estimation of Aliskiren in Pharmaceutical Dosage Form by RP-HPLC
Yadav Nita1* and Goyal Anju2
1Department of Pharmacy, SRMSCET, India
2B N Institute of Pharmaceutical Sciences, India
Submission: February 10, 2018; Published: February 23, 2018
*Corresponding author: Nita Yadav, Department of Pharmacy, SRMSCET, Bareilly, India, Tel: +919458702598; Email: raj_neetu78@rediffmail.com
How to cite this article: Yadav N, Goyal A. Method Development and Validation for the Estimation of Aliskiren in Pharmaceutical Dosage Form by RPHPLC. Organic & Medicinal Chem IJ. 2018; 5(3): 555663. DOI: 10.19080/OMCIJ.2018.05.555663
Abstract
A simple, sensitive and rapid reverse phase high performance liquid chromatographic method was developed for the estimation of Aliskiren in tablet dosage form. A C8 Chromasil column (150 x 4.6 mm, 5μm particle size) was used as a stationary phase with a mobile phase containing a mixture of phosphate buffer, methanol and ACN in ratio of 55:10:35, v/v. The flow rate was 1.0 mL/min. The effluent was monitored at 220 nm and eluted at 2.342 min. Calibration curve was plotted with a range from 30-150 μg/ml for Aliskiren and the correlation was found to be 0.999. The accuracy range was found between 100.06-100.43%. The % RSD values for both intraday and interday precision were less than 2.0. The limit of detection (LOD) and limit of quantification (LOQ) were found to be 2.335μg/ml and 7.077μg/ml respectively. The assay was validated for the parameters like system suitability, precision, accuracy, and robustness parameters. The proposed method can be useful for the routine determination of Aliskiren in pharmaceutical dosage form.
Keywords: Aliskiren; Calibration curve; RP-HPLC; Validation; ICH guidelines
Abbreviations: LOD: Limit of detection; LOQ: Limit of quantification; ALH: Aliskiren hemifumarate
Introduction
Hypertension is a common chronic disease that leads to significant cardiovascular morbidity and mortality worldwide. Blood pressure control is important for the management or prevention of cardiovascular diseases and their complications [1]. New antihypertensive agents with a different mechanism of action may play an important role in the optimal management of hypertension. The key factor in the development of any new antihypertensive agent is its potential to effectively control blood pressure [2]. Aliskiren hemifumarate (ALH) is chemically described as (2S, 4S, 5S, 7S)-N-(2-methylpropyl) 5-amino-4- hydroxy-2, 7-diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy) phenyl] octamide hemifumarate, a renin inhibitor, widely used for the treatment of essential hypertension (Figure 1). ALH is a white to slightly yellowish crystalline powder with a molecular weight of 609.8 (free base 551.8). It is soluble in phosphate buffer, n-octanol, and highly soluble in water. ALH is the first of a new class of antihypertensive agents. ALH blocks the renin system at its rate-limiting step by directly inhibiting the catalytic activity of renin, thereby reducing generation of angiotensin I and angiotensin II [3,4].

In the present study, we developed and validated a rapid and sensitive HPLC method to determine Aliskiren in pharmaceutical formulations in a run-time of 2.335 min. The short run time enables efficient analysis of large number of formulation samples obtained for regular qualitative control analysis. To the best of our knowledge, this is the first report of a fully validated HPLC method for the quantification of Aliskiren in formulations. This method was successfully applied to the estimation studies of Aliskiren in marketed products. Existing literature reveals that there are only few methods for the assay of Aliskiren in bulk and dosage forms [5-8]. Hence an attempt has been made to develop new simple, reliable, and reproducible, isocratic RP-HPLC methods to estimate the Aliskiren in bulk and pharmaceutical formulation with good precision, accuracy, linearity and reproducibility respectively. Hence in present work, we developed a simple, rapid and inexpensive liquid chromatographic method for the analysis of Aliskiren in pure and pharmaceutical dosage form. The proposed HPLC method is validated using standard ICH guidelines [9].
Materials and Methods
The pure drug of Aliskiren was procured as gift sample from Swapnroop Drugs and Pharmaceuticals, Aurangabad, Maharashtra. All the chemicals were used analytical grade. HPLC grade acetonitrile (ACN), methanol and water from Merck Pharmaceutical Private Ltd., Mumbai and India were used. Rasilez tablets-Novartis (each tablet contains 150 mg of aliskiren) were purchased from local market. Membrane filters 0.45 μm and 0.2 μm were procured from Millipore Pvt. Ltd. Bangalore, India.
Instrumentation
LC system used consists of pump (Model Shimadzu; LC-10 AT VP) with universal loop injector (Rheodyne 772 5 i) of injection capacity 20 μl. Detector consists of photodiode array detector SPD-10 AVP. C8 Chromasil column (250 x 4.6 mm, 5μm particle size) was used. The HPLC system was equipped with LC solution software.
Preparation of the Mobile Phase
The mobile phase was prepared by mixing of phosphate buffer (2.72 gm pot. dihydrogen phosphate transferred in 1000 ml volumetric and adjusted pH 6.5 with 0.1N sodium hydroxide), methanol and ACN in ratio of 55:10:35, v/v. It was filtered through 0.2 μm membrane filter and then sonicated for degassing. It was pumped from the respective solvent reservoirs to the column at a flow rate of 1.0 ml/min. prior to the injection of the drug solution; the column was equilibrated for at least 30 min with the mobile phase flowing through the system. The eluent was monitored at 220 nm.
Preparation of Stock and Working Standard Solutions
Accurately weighed powder equivalent to 30 mg of Aliskiren was transferred in a 100 mL clean, dry volumetric flask and mobile phase was added and sonicated to dissolve. The volume was made up to the mark with mobile phase to prepare 300 μg/ mL stock solutions. 3mL of this solution was transferred into 10mL volumetric flask and volume was made up to the mark with mobile phase to prepare 90 μg/mL working standard solution.
Preparation of Sample solution
For the estimation of Aliskiren in the tablet formulation, 20 tablets (label claim 150 mg) were accurately weighed and the average weight per tablet was calculated. The tablets were crushed and finely powdered in glass mortar. Powder equivalent to 30 mg of Aliskiren was accurately weighed and transferred into a 100 mL volumetric flask and sonicated to dissolve. The volume was made up to the mark with mobile phase, mixed well to prepare 300 μg/mL stock solutions. The solution was filtered using 0.2 μm membrane filter and degassed by sonication. 3 mL of this solution was transferred into 10mL volumetric flask and volume was made up to the mark with mobile phase to prepare 90 μg/mL test solution. The resulting solution was used as the sample solution for chromatographic analysis. After setting the chromatographic conditions and stabilizing the instrument to obtain a steady baseline, the sample solution was injected three times and the peak areas were recorded.
Chromatographic Conditions
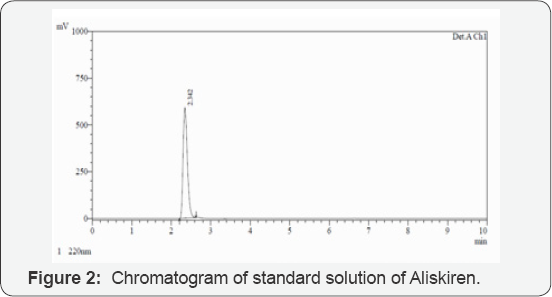
The chromatographic separation was performed using a C8 Kromasil column (150mm X 4.6mm i.d, 5 μm particle size). The mobile phase consists of a mixture of phosphate buffer (pH 6.5), methanol and ACN in a ratio of 55:10:35, v/v. The mobile phase was set at a flow rate of 1 mL/min and the analytes were monitored at 220 nm. The column was maintained at 25°C temperature and injected volume was 20 μl. The total run time was 10 min. The mobile phase was filtered through 0.2 μm membrane filter prior to use. A typical chromatogram of Aliskiren is shown in (Figure 2).
Method Validation
The method was validated according to ICH guidelines to study system suitability, linearity, precision, accuracy and robustness, limit of detection (LOD) and limit of quantitation (LOQ).
System suitability: System suitability test parameters for Aliskiren for the developed method are reported in (Table 1). % RSD for tailing factor, theoretical plate count, peak area and retention time for Aliskiren were found to be within the limit of 2%, which indicates suitability of the system. The number of theoretical plates and tailing factor were found within the acceptance criteria of >2000 and ≤2.0, respectively, indicating good column efficiency and optimum mobile phase composition.
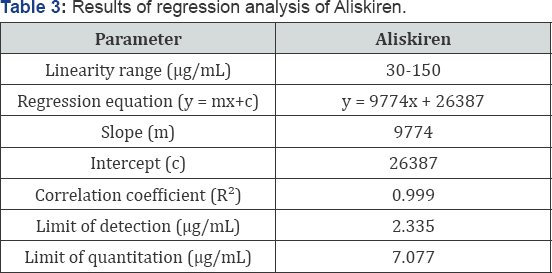
Linearity: Linearity of this method was evaluated by linear regression analysis and calculated by least square method and studied by preparing standard solutions of Aliskiren at different concentration levels. Peak area of resulting solutions was measured and the calibration curve was plotted between peak area and concentration of the drug (Figure 3). The response was found to be linear in the range 30-150 μg/ml for Aliskiren.The data was given in (Tables 2 & 3).

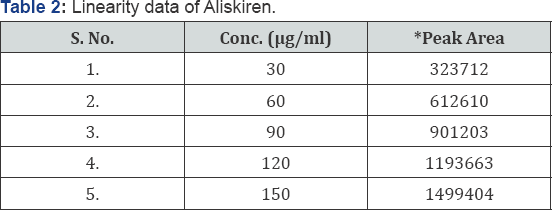
*Peak area mean of three replicates.
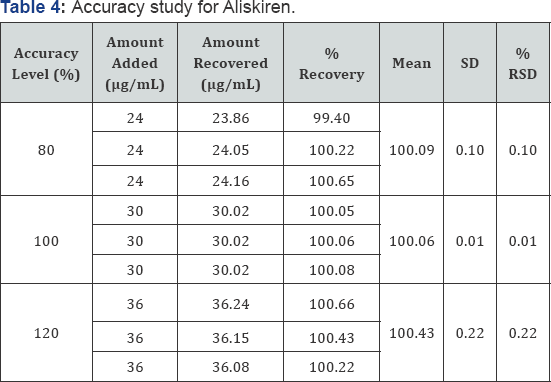
Accuracy /Recovery: Accuracy is the degree of agreement between a measured value and the accepted reference value. The accuracy of the method was tested by triplicate samples at 3 different concentrations equivalent to 80%, 100% and 120% of the active ingredient, by adding a known amount of Aliskiren standard to a fixed amount of the pre-analysed sample of Aliskiren. The recovered amount of Aliskiren, % recovery and %RSD of each level is calculated to determine the accuracy. The data was given in (Table 4).
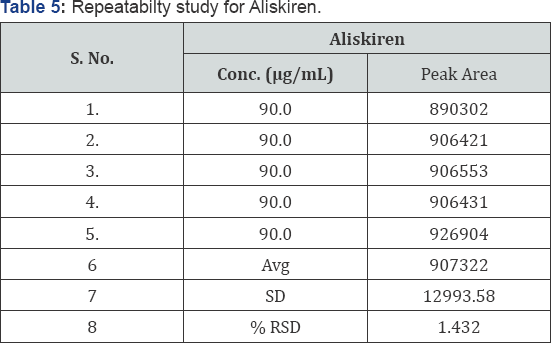
Repeatability: Five sample solutions of the same concentration were prepared and injected into the HPLC system as per test procedure. The results were given in (Table 5).
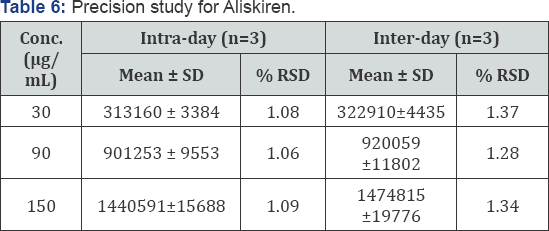
Precision (Day to Day variability): Intra-day precision was investigated by replicate applications and measurements of peak area for Aliskiren for three times on the same day under similar conditions. Inter-day precision was obtained from % RSD values obtained by repeating the assay three times on different days. The percent relative standard deviation (% RSD) was calculated. The results were given in (Table 6).
Limit of detection and Limit of Quantitation: LOD and LOQ were calculated from the average slope and standard deviation from the calibration curve as per ICH guidelines.
Robustness: Robustness of an analytical method is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters and provide an indication of its reliability during normal usage. Robustness can be determined by analysis of solution by changing physical parameters like composition of mobile phase and flow rate, In order to measure the extent of method robustness, the parameters were interchanged while keeping the other parameters unchanged and in parallel, the chromatographic profile was observed and recorded. The results of small variations in these parameters were shown in (Table 7).

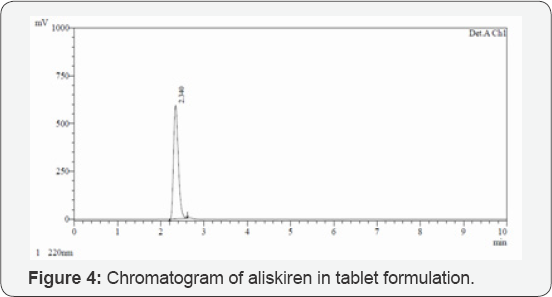
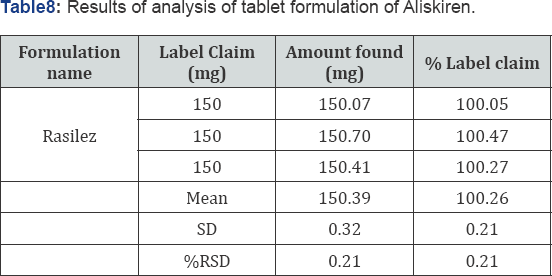
Assay: The developed method was applied to the assay of Aliskiren tablets. From the peak areas the amount of drug present in tablet was estimated. The drug content was calculated as an average of three determinations and assay results were shown in (Table 8). The results were very close to the labeled value of commercial tablets. The representative chromatogram of Aliskiren is shown in (Figure 4).
Results and Discussion
A reverse-phase HPLC method was proposed as a suitable method for the determination of Aliskiren in tablet dosage form. The chromatographic conditions were optimized by changing the mobile phase composition. Different ratios were experimented to optimize the mobile phase. Finally, mixture of phosphate buffer, methanol and ACN in ratio of 55:10:35, v/v was used as mobile phase, which showed good resolution of Aliskiren peak. The wavelength of detection selected was 220 nm, as the drug showed sharp and better peak shape at this wavelength. By developed method the retention time of Aliskiren was about 2.342 min. In the present developed HPLC method, the standard and sample preparation required less time and no tedious extraction were involved. A good linear relationship (r2=0.999) was observed between the concentration range of 30-150 μg/mL. The assay of Aliskiren in tablet dosage forms was found to be 100.26%. The statistical analysis of data and the drug recovery data showed that the method was simple, rapid, economical, sensitive, precise and accurate. It can thereby easily adopt for routine quality control analysis. The results of this analysis confirmed that the developed method was suitable for determination of drug in pharmaceutical formulation has no interference of additives. Hence the developed method can be applied for estimation of Aliskiren in marketed formulation.
Conclusion
The developed method is rapid, accurate and sensitive. It makes use of fewer amounts of solvents and change of set of conditions requires a short time. This method can be suitably analyzed for the routine analysis of Aliskiren in tablet dosage form. It does not suffer from any interference due to common excipients present in pharmaceutical preparation and can be conveniently adopted for quality control analysis. The above proposed method obviates the need for any preliminary treatment and is the method could be of use for process development as well as quality assurance of Aliskiren.
Acknowledgement
The authors express their sincere gratitude to Swapnroop Drugs and Pharmaceuticals, Aurangabad, Maharashtra, for providing the pure drug sample of Aliskiren and are also thankful to colleagues and authorities of Pacific University, Udaipur who helped us in this work.
References
- Lam S, Choy M (2007) Aliskiren: an oral renin inhibitor for the treatment of hypertension. Cardiol Rev 15(6): 316-323.
- Schmieder RE (2006) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. J Renin Angiotensin Aldosterone Syst 7: S16.
- Daugherty KK (2008) Aliskiren. Am J Health Syst Pharm 65(14): 13231332.
- Pranay Wal, Ankita Wal, Awani KR, Dixit A (2011) Aliskiren: An orally active rennin inhibitor. J Pharm Bioallied Sci 3(2): 189-193.
- Sangoi W, Leonardo TS, Isabel FD, Rolim MB (2010) Development and validation of an UV-spectrophotometric method for the determination of aliskiren in tablets. Qui'm Nova 33(6): 123-130.
- Ezzeldin MI, Shokry E, Fouad MA, Elbagary RI (2013) Application of chromatographic and spectrophotometric methods for the analysis of selected antihypertensive combinations. Inter J of Analyt Pharma Biomed Scie 2(3): 6-15.
- Wrasse Sangoi M, Sangoi MS, Oliveira PR, Secretti LT, Rolim CMB (2011) Determination of Aliskiren in Tablet Dosage Forms by a Validated Stability-indicating RP-LC Method. J Chromatogr Sci 49(2): 170-175.
- Wrasse Sangoi MdS, Oliveira PRd, Todeschini V, Rolim CMB (2011) Simultaneous determination of aliskiren and hydrochlorothiazide from their pharmaceutical formulations by monolithic silica HPLC column employing experimental designs. J Liquid Chromatogr Related Technol 34(17): 1976-1996.
- ICH, Q2 (R1) (2005) Harmonized Tripartite Guideline, Validation of Analytical procedures Text and methodology. International Conference on Harmonization (ICH), Geneva, Switzerland.






























