Promising Antibacterial Activity of Simple Schiff Bases
Ragavi vadivel1, Reshma Jayakumar1 and Nallamuthu Ananthi2*
1PSGR Krishnammal College for Women, India
2Department of Chemistry, Karunya Institute of Technology and Sciences, India
Submission: February 10, 2018; Published: February 23, 2018
*Corresponding author: Nallamuthu Ananthi, Department of Chemistry, Karunya Institute of Technology and Sciences, Coimbatore, Tamil Nadu, India, Tel: 99941 49762, Email: write2ananthi@gmail.com
How to cite this article: Ragavi vadivel, Reshma Jayakumar, Nallamuthu Ananthi. Promising Antibacterial Activity of Simple Schiff Bases. Organic & Medicinal Chem IJ. 2018; 5(3): 555662. DOI: 10.19080/OMCIJ.2018.05.555662
Abstract
This review comprised the recently reported novel Schiff bases and their metal complexes which were found to have medicinal and biological activities in terms of antibacterial, anti-fungal, anti-malarial, anti-proliferative, and anti-inflammatory and anti cancer activities. The structure activity relationship for the potential biological and medicinal activities of the Schiff bases was also discussed.
Introduction
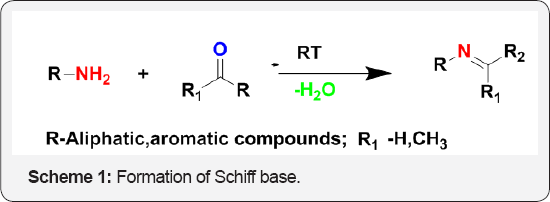
Schiff base are the compound containing azomethine group (-HC=N-). They are the condensation products of ketones (or) aldehydes with primary amines (Scheme 1). They were first reported by Hugo Schiff in 1864. Formation of Schiff base generally takes place under acid or base catalysis or with heat. Schiff bases are some of the most widely used organic compounds. Schiff bases have also been shown to exhibit a broad range of biological activities, including anti-fungal, anti-bacterial, anti- malarial, anti-proliferative, anti-inflammatory activities. Schiff base ligands which usually contain O and N donor atoms have played an important role in coordination chemistry and recently considerable attention has been paid to nitrogen and other donors. Schiff bases are active against a wide range of organisms since they play an important role in living organisms, such as decarboxylation, transamination and C-C bond cleavage.
Many Schiff base complexes show excellent catalytic activity in various reactions. Over the past few years, there have been many reports on their applications in homogeneous and heterogeneous catalysis. The high thermal and moisture stabilities of many Schiff base complexes were useful attributes for their application as catalysts in reactions involving at high temperatures. The activity is usually increased by complexation therefore, to understand the properties of both ligands and metal can lead to the synthesis of highly active compounds. The influence of certain metals on the biological activity of these compounds and their intrinsic chemical interest as multi dentate ligands has prompted a considerable increase in the study of their coordination. Development of a new chemotherapeutic Schiff bases and their metal complexes is now attracting the attention of medicinal chemists.
Increase in the biological activity was reported by the implementation of transition metal complexes of Schiff bases. Synthesis, characterization and structure activity relationship (SAR) of Schiff bases are been studied worldwide. Several studies showed that the presence of a lone pair of electrons in sp2 hybridized orbital of nitrogen atom of the azomethine group is of considerable chemical and biological importance [1]. They interfere in normal cell processes by the formation of a hydrogen bond between the active centers of cell constituents and sp2 hybridized nitrogen atom of the azomethine group [2]. Therefore, it will be interesting to study the antibacterial activity of Schiff base.
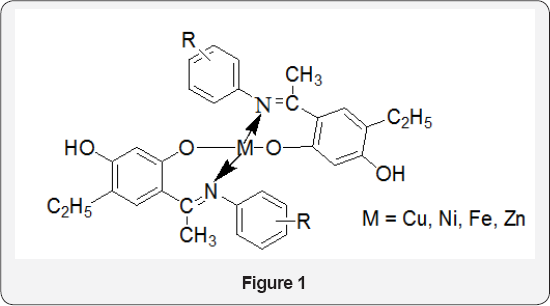
Copper, Nickel, Iron and Zinc complexes of Schiff bases (Figure 1) was synthesized. The metal complexes were screened for antibacterial activity against some clinically important bacteria, such as Pseudomonas aeruginosa, Proteus vulgaris, Proteus mirabilis, and Klebsiella pneumoniae and Staphylococcus aureus. Antibacterial activity was determined by the Agar Ditch technique using DMF (polar) and 1, 4-dioxane (nonpolar) as solvents. The Schiff bases showed greater activity than their metal complexes. The metal complexes showed differential effects on the bacterial strains investigated and the solvent used, suggesting that the antibacterial activity is dependent on the molecular structure of the compound, the solvent used and the bacterial strain under consideration. Amongst the four metals, zinc complex showed the best antibacterial activity followed by the iron complex in 1, 4-dioxane. Iron complex showed the best antibacterial activity in DMF [3].
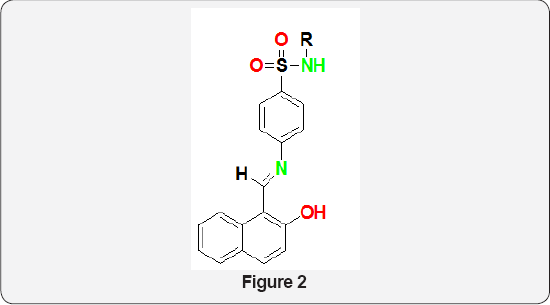
Four novel metal complexes of Co(II), Ni(II), Cu(II) and Zn(II) were synthesized from Schiff base derived from amoxicillin and picolinaldehyde by NK Chaudhary et al. [4]. The ligand and metal complexes were screened for antibacterial potency against four human pathogenic clinical strains of bacteria and the data revealed their promising antibacterial activity. Schiff bases of type (Figure 2) were synthesized from the condensation of 2- (hydroxyl naphthaldehyde and sulfonamides (sulfathiazole, sulfa pyridine, sulfadiazine, sulfamerazine and sulfa-guanidine). Antimicrobial activities of these Schiff bases have been evaluated against certified and resistant Gram positive (Staphylococcus aureus, Enterococcus facelis) and Gram negative (Streptococcus pyogenes, Salmonella typhi, Shigelladysenteriae, Shigellaflexneri, Klebsiella pneumonia) pathogens. Performance of these Schiff base against the resistant pathogens are better than standard stain and MIC (Minimum Inhibitory Concentration) data lie 32-128 μg/ml while parent sulfonamides are effectively inactive (MIC >512 μg/ml). The DFT optimized structures of the Schiff bases have been used to accomplish molecular docking studies with DHPS (dihydropteroate synthase) protein structure to establish the most preferred mode of interaction [5].
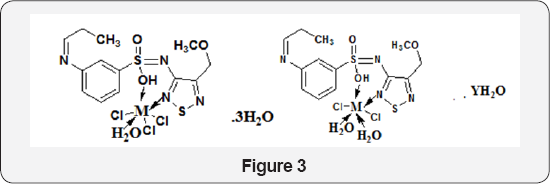
A novel Schiff base was derived from simple condensation of 2-hydroxy-6-isopropyl-3-methyl benzaldehyde and 1, 2 -diaminopropane in 2:1 M ratio. Mn, Co and Ni complexes of the Schiff base have been prepared by Samina K Tadavi et al. [6]. All the synthesized compounds were screened for their antimicrobial activity and for DNA cleavage activities. Fe(III), Co(II) and Ni(II) complexes (Figure 3) of sulphametrole derived Schiff bases were synthesized by Mohamed et al. [7]. The metal complexes good antibacterial activity against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus pyogones. The complexes caused inhibition for Escherichia coli. The importance of this lies in the fact that these complexes could be applied fairly in the treatment of some common diseases caused by Escherichia coli. The complexes were found to inhibit Gram-positive bacterial strains (Staphylococcus Pyogones and Pseudomonas aeruginosa).
A new series of oxovanadium (IV) complexes have been designed and synthesized from a new class of triazole Schiff bases derived from the reaction of 3, 5-diamino-1,2, 4-triazole with 2-hydroxy-1-naphthaldehyde, pyrrole- 2- carboxaldehyde, pyridine-2-carboxaldehyde and acetyl pyridine-2-carboxaldehyde, respectively by Zahid H Chohan et al. [8]. The biological activity of the complexes have been studied against four Gram-negative (Escherichia coli, Shigellaflexenari, Pseudomonas aeruginosa, Salmonella typhi) and two Gram- positive (Staphylococcus aureus, Bacillus subtilis) bacterial strains, in vitro antifungal activity was studied against Trichophytonlongifucus, Candida albican, Aspergillusflavus, Microscopumcanis, Fusariumsolani and Candida glaberata. The Schiff bases showed weaker to significant activity against one or more bacterial and fungal strains. In most of the cases higher activity was exhibited upon coordination with vanadium (IV) metal. Brine shrimp bioassay was also carried out for in vitro cytotoxic properties against Artemiasalina.
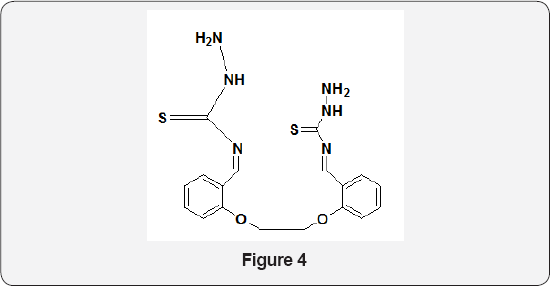
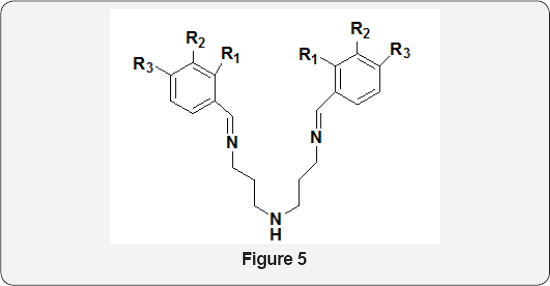
Novel Schiff bases of type (Figure 4) were reported by Ehab M Zayed et al. [9]. Their biological activities have been tested in vitro against Escherichia coli, Proteus vulgaris, Bacillissubtilies and Staphylococcus aurous bacteria in order to assess their antimicrobial potential. DNA binding, cleavage and cytotoxicity characteristics of a novel Schiff base ligand 3- (benzothiazol-2- yliminomethyl)-naphthalen-2-ol and its ruthenium(II) complexes have been investigated. Ruthenium (II) complexes show more binding ability than the ligand. In vitro cytotoxicity study of the ligand and the complexes exhibited antitumor activity against tumor cells [10]. A total of 24 Schiff base compounds were synthesized using a simple approach with cinnamaldehyde derivates and 8 amino acids by Wang et al. [11]. The antimicrobial activities of these compounds were evaluated against Aspergillusniger, Penicilliumcitrinum, Escherichia coli and Staphylococcus aureus. Results from the structure activity relationship suggest that both -p-Cl on benzene ring of cinnamaldehyde and the number of -COOK of amino acid salts significantly contributed to antimicrobial activity. NK Chaudhary et al. [12]. prepared six Schiff bases by reacting 3, 3' -diaminodipropylamine with different benzaldehyde derivatives. The prepared compounds were evaluated for in vitro antimicrobial activity against a number of pathogenic Gram- positive and Gram-negative bacteria. These compounds showed bacteriostatic rather than bactericidal activities against Gram positive and Gram-negative bacteria. In addition, compound (Figure 5) exhibited significant anticandida activity with the minimum inhibitory concentration of 24 lg/ml and is, therefore, considered as a promising and potential antifungal agent.
A Novel Schiff base, 3-(((4-chlorophenyl)imino)methyl) benzene-1,2-diol was successfully synthesized along with a structurally similar Schiff base 3-(((4-bromophenyl)imino) methyl)benzene-1,2-diol. Both the Schiff bases were used to synthesize their zinc (II) and cobalt (II) complexes. These compounds were screened for enzyme inhibition, antibacterial, cytotoxic and invivo antidiabetic activities. Results indicate that zinc complex is a good inhibitor of alkaline phosphatase enzyme and possess highest potential against diabetes, blood cholesterol level and cancer cells [13]. New ether based Schiff bases were synthesized from 5-chloro-2-hydroxy benzaldehyde and primary amines (1-amino-4-phenoxybenzene, 4-(4-aminophenyloxy) biphenyl, 1-(4-aminophenoxy) naphthalene and 2-(4-aminophenoxy) naphthalene). From these Schiff bases copper (II) complexes were synthesized. The synthesized Schiff bases and copper (II) complexes were further assessed for various biological studies. In DNA protection assay significant protection behavior was exhibited by simple ligand molecules while copper (II) complexes showed neutral behavior [14].
A Schiff base 1-((3-nitrophenylimino) methyl) naphthalen-2- olate and its Zn(II) and Co(II) metal complexes were successfully synthesized. All these prepared compounds were screened for enzyme inhibition, antibacterial, cytotoxic and invivo antidiabetic activities and found active against one or other activity [15]. A new Schiff base 2,4-diiodo-6-((2-phenylaminoethylimino) methyl)phenol and its Cu(II), Ni(II), Co(II), Mn(II) and Zn(II) metal complexes have been synthesized. Metal complexes showed good antibacterial activities. Elham S Aazam et al. [16]. Synthesized 2, 3-bis-[(3-ethoxy-2-hydroxybenzylidene) amino] but-2-enedinitrile Schiff base ligand and its corresponding copper and nickel complexes. The antibacterial activity of the copper complexes were screened against Escherichia coli, which demonstrated Minimum Inhibitory Concentration and Minimum Bactericidal Concentration values lower than those values of the commercially prepared complexes [17]. Three mononuclear complexes, one Cu(II) complex and two Zn(II) complexes have been synthesized through treating the corresponding metal salts with Schiff bases of trimethylsilyl-propyl-p-aminobenzoate with salicylaldehyde) and o-vanillin. The antifungal and antibacterial properties of the prepared compounds against Aspergillus fumigatus ATCC 66567, Penicillium chrysogenum ATCC 20044, Fusarium ATCC 20327, Bacillus sp. ATCC 31073, Pseudomonas sp. ATCC 15780 were evaluated. Both Schiff bases and metal complexes showed better antimicrobial activity compared to the standard compounds Caspofungin and Kanamyci [18].
Mostafa Y Nassar et al. [19]. Synthesized Co (II) and Ni(II)- triazole Schiff base complexes by refluxing the prepared triazole Schiff bases with CoCl2•6H2O or NiCl2•6H2O. The prepared Co(II) and Ni(II) complexes were screened for antifungal (Candida albicans and Aspergillus flavus) and antibacterial (Staphylococcus aureus and Escherichia coli) activities. Schiff bases (2-(1-hydrazonoethyl) phenol), (2, 4-dibromo 6-(hydrazonomethyl) phenol) and (2-(hydrazonomethyl) phenol) were prepared as new hydrazone compounds via condensation reactions. Their biological activities have been tested in vitro against Escherichia coli, Proteus vulgaris, Bacillissubtilies and Staphylococcus aurous bacteria in order to assess their anti microbial potential [20]. Hydrazone hesperetin Schiff base N-[(±)-[5, 7-dihydroxy-2-(3-hydroxy- 4-methoxy-phenyl)chroman-4-ylidene]amino]benzamide has been synthesized by Elizbieta Lodyga Chruscinska et al. [21]. The corresponding Cu(II) complexes were also synthesized. The studies on DNA interactions, antimicrobial and cytotoxic activities have been undertaken to gain more information on the biological significance of the complexes.
Jaganathan Venkatesan et al. [22]. Synthesized new sulfonamide Schiff bases namely, 4-(benzylideneamino) benzenesulfonamide and 4-((4-methylbenzylidene)amino) benzenesulfonamide from benzaldehyde. The Schiff bases showed good antibacterial activity. New Zn(II) binary complex was synthesized from well defined Schiff base, the antimicrobial properties of the material were demonstrated against Gram- positive (Staphylococcus aureus, Bacillus subtilis, Bacillus cereus) and negative (Escherichia coli, Pseudomonas aeruginosa, Xanthomonas campestris) bacteria using modified agar diffusion methods. All zinc loaded nanoparticles were less antimicrobially active than zinc compounds alone, as encapsulation controls their release, thereby attenuating their antimicrobial activity [23]. New Schiff base ligand was prepared via condensation of o-phthaldehyde and 2-aminobenzoic acid in 1:2 ratio. Metal complexes were prepared and screened for biological activity. The biological data showed that the metal complexes to be more potent/antibacterial than the parent Shciff base ligand against one or more bacterial species [24]. Newly synthesized Schiff base 2-((2-((2-(allylthio)-1-carboxyethyl)imino)ethylidene) amino)-4-(methylthio)butanoic acid have good antioxidant property, which was revealed by reduced oxidative stress and enhanced functions of the endogenous antioxidative against CCl4 intoxication. It also protects the functional activities of the various mitochondrial tricarboxylic acid cycle and oxidative phosphorylation enzymes [25].
Three Schiff base compounds of N'-substituted benzohydrazide and sulfonohydrazide derivatives, N'-(2-hydroxy-3-methoxy benzyl idene)-4-tert-butyl- benzohydrazide, N'-(5-bromo-2-hydroxybenzylidene)-4- tert-butylbenzohydrazide and N'-(2-hydroxy-3-methoxybenzylidene)-4-methylbenzenesulfonohydrazide were synthesized. The compounds have been screened for their biological activities including, antibacterial, antifungal, cytotoxic, enzymatic activities. The synthesized compounds were also found to be effective against alkaline phosphatase enzyme. They also show significant to good antimicrobial activity against six bacterial and five fungal strains. The MIC (Minimum Inhibitory Concentration) for antibacterial activity ranges from 1.95-500 μg/mL [26]. New Schiff bases from sulfanilamide,3- fluorosulfanilamide or 4-(2-aminoethyl)-benzenesulfonamide were synthesized by Mariangela Ceruso et al. [2 7].Their antifungal, antibacterial and antiprotozoan activities have been determined against the pathogenic fungus Cryptococcus neoformans, the bacterial pathogen Brucellasuis and the protozoan parasite Leishmaniadonovanichagasi, responsible for Leishmaniasis. The results of these inhibition studies show that all three enzymes were efficiently inhibited by sulfonamide Schiff bases.
Omyma AMA et al. [28]. Synthesized Schiff bases, 2-[(pyridin- 2-ylmethylidene) amino]-6-aminopyridine and 2-[(pyridin- 2-ylmethylidene)amino]phenol. Cr, Mo, and W complexes of these Schiff basees were prepared. The catalytic activity of the complexes towards hydrogen peroxide decomposition reaction was investigated. Both the ligands and their complexes have been screened for antibacterial activities. Novel Schiff base ligand was prepared via condensation of benzyl and triethylenetetraamine. The complexes are prepared using Mn(II), Co(II), Ni(II), Cu(II), Zn(II), Cd(II) salts. The metal complexes were screened for its antibacterial activity. The activity data show that the metal complexes have antibacterial activity more than the parent Schiff base ligand [29]. New Azo Schiff base compounds have been synthesized from the reaction of m-hydroxy benzoic acid with 1,5-dimethyl-3-[2-(5-methyl-1H-indol-3-yl)- ethylimino]-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylamine and with 3-[2-(1H-indol-3-yl)-ethylimino]-1,5-dimethyl-2-phenyl- 2,3-dihydro-1H-pyrazol-4-ylamine. The free ligands and their complexes were screened for their biological activity against bacterial species, two Gram positive bacteria (Bacillus subtilis and Staphylococcus aureus) and two Gram negative bacteria (Escherichia coli and Pseudomonas aeruginosa) [30].
Some new water-soluble complexes (M = Zn, Cu, Ni, Mn) of Schiff base N,N'-bis(5-sulfosalicyliden)-3,4- diaminobenzophenone were synthesized. The synthesized Schiff base and its metal complexes were screened for antibacterial activity. The growth inhibitory effects of the complexes toward the cancer cell line were measured [31]. New acyclic Schiff base and its chromium (III) complexes were synthesized by microwave irradiation method. The complexes were screened for antimicrobial activity and showed inhibitory effect towards the Gram positive and Gram negative bacteria [32]. Nadia Ribeiro et al. [33]. Synthesized Schiff base by the condensation of 5-methyl-1H-pyrazole-3-carbohydrazide with different aromatic aldehydes-pyridoxal, salicylaldehyde, 3-methoxy-2- hydroxybenzaldehyde, 3-ethoxy-2- hydroxybenzaldehyde and 2 hydroxynaphthene-1-carbaldehyde. The corresponding copper complexes were prepared. Metal complexes and Schiff base were screened for antibacterial studies. The growth inhibitory effects of the complexes toward the cancer cell line were measured.
Deepak Kumar et al. [34]. Synthesized benzyl-[3- (benzylamino-methyl)-cyclohexylmethyl]-amine derivatives with different substitution pattern on the aromatic ring and evaluated for their antibacterial activity against Gram-positive and Gram-negative bacterial strains. Most of the compounds exhibit potent activity against Pseudomonas aeruginosa and Staphylococcus epidermidis with minimum inhibitory concentration values ranging from 0.002 to 0.016 μg/mL. A novel Schiff base ligand was prepared by the condensation of 3, 4-(methylenedioxy) aniline and 5-bromo salicylaldehyde. The corresponding Zn(II), Cd(II), Ni(II), Cu(II), Fe(III), Co(II), Mn(II) Hg(II), and Ag(I) complexes were prepared. The in vitro antimicrobial effects of the synthesized compounds were tested against five bacterial and three fungal species by well diffusion method. Metal complexes show more biological activity than the Schiff base [35].
Rabiul Hasan et al. [36]. Synthesized nickel (II) complexes of the dibasic tridentate Schiff bases. The bio efficacy of the prepared complexes has been examined against the growth of bacteria and fungi in vitro to evaluate their antimicrobial potential. Gehad et al. [37]. Synthesized metal complexes from 2.6- pyridinedicarboxaldehydebis (p-hydroxyphenylimine) and 2.6- pyridinedicarboxaldehydebis (o-hydroxyphenylimine). Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes of the ligands were also prepared. The synthesized ligands, and their metal complexes were screened for their antibacterial activity against bacterial species, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Fungi (Candida). The activity data show that the metal complexes to be more potent/antibacterial than the parent organic ligands against one or more bacterial species.
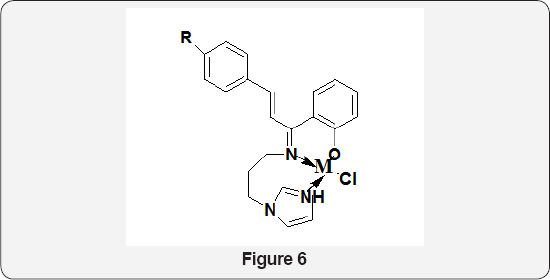
Mehmet Aslantas et al. [38]. synthesized trans-N,N'-bis[(2,4- dichlorophenyl) methylidene] cyclohexane-1,2-diamine and its copper(II), nickel(II) and palladium(II) metal complexes. The antimicrobial activity studies of the ligand and its metal complexes were carried out on various bacteria and fungi [39]. Metal complexes of Schiff base prepared by the condensation of 4- aminoantipyrine and 2-aminophenol. The synthesized ligand, in comparison to its metal complexes was screened for their antibacterial activity against bacterial species, Escherichia coli, Pseudomonas putida, Exiguobacteriumacetylicum and Bacillus simplex. The activity data show that the metal complexes to be more potent/antibacterial than the parent Shciff base ligand against one or more bacterial species. Kalanithi M et al. [40]. synthesized tridentate Co(II), Ni(II), Cu(II) and Zn(II) complexes of chalcone based ligands 2-[1-(3-(1H-imidazol-1-yl) propylimino)-3-(phenylallyl)]phenol, 2-[1-(3-(1H-imidazol-1- yl)propylimino)-3-p-tolylallyl]phenol, and 2-[1-(3-(1H-imidazol- 1- yl)propylimino)-3-4-nitrophenylallyl]phenol (Figure 6) [41]. The antimicrobial activity of the ligands and the metal complexes against the species Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Candida albigans and Aspergillusniger has been carried out and compared. The electrochemical behavior of copper (II) complex was studied using cyclicvoltammetry.
Conclusion
In this review, an attempt has been made to cover the recently reported Schiff bases and their metal complexes which are possessing medicinal and biological activities. Most of the Schiff bases reported were simple to synthesize and contains numerous biological applications. Still there is hope to develop novel Schiff bases and their metal complexes which can be applied in medicinal field.
References
- GT Selvan, V Chitra, Enoch IVMV, PM Selvakumar (2018) Development of fluorescent chemosensor towards sensing and separation of Mg2+ ions in chlorophyll and hard water. New J of Chem 42(2): 902-909.
- S Patai, KN Venugopala, BS Jayashree (1970) The Chemistry of Carbon- Nitrogen Double Bond. Interscience, New York, USA, pp. 149-180.
- K Vashi, HB Naik (2004) Synthesis of carboxamides of 2'-amino 4'-(6-bromo-3-coumarinyl) thiazole as analgesic and anti inflammatory agents. Indian J Heterocyclic Chem 12(3): 307-310.
- R Nair, A Shah, S Baluja, S Chandai (2006) Synthesis and antibacterial activity of some Schiff base complexes. J Serb Chem Soc 71(7): 733744.
- K Chaudhary, P Mishra (2017) Bioactivity of some divalent M (II) complexes of penicillin based Schiff base ligand: Synthesis, spectroscopic characterization, and thermal study. J Saudi Chemical Society p. 1-25.
- S Mondal, SM Mandal, TK Mondal, C Sinha (2017) Spectroscopic characterization, antimicrobial activity, DFT computation and docking studies of sulfonamide Schiff bases. J Molecular Structure 11(27): 557567.
- SK Tadavi, AA Yadav, RS Bendre (2018) Synthesis and characterization of a novel schiff base of 1,2-diaminopropane with substituted salicyaldehyde and its transition metal complexes: Single crystal structures and biological activities. Transition Metal Chemistry 40(7): 715-722.
- AM Abu-Dief, IMA Mohamed (2015) A review on versatile applications of transition metal complexes incorporating Schiff bases 4(2): 119133.
- ZH Chohan, SH Sumrra, MH Youssoufi, TB Hadda (2010) Metal based biologically active compounds: Design, synthesis, and antibacterial/ antifungal/cytotoxic properties of triazole-derived Schiff bases and their oxovanadium(IV) complexes. European Journal of Medicinal Chemistry 45(7): 2739-2747.
- EM Zayed, ME Desawy (2015) Preparation and structure investigation of novel Schiff bases using spectroscopic, thermal analyses and molecular orbital calculations and studying their biological activities. 134: 155-164.
- HFA El-Halim, GG Mohamed, EAM Khalil (2017) Synthesis, spectral, thermal and biological studies of mixed ligand complexes with newly prepared Schiff base and 1, 10-phenanthroline ligands. Journal of molecular structure 1146: 153-163.
- H Wang, H Yuan, S Li, Z Li (2016) Synthesis, antimicrobial activity of Schiff base compounds of cinnamaldehyde and amino acids. Bioorganic & Medicinal Chemistry Letters 26(3): 809-813.
- SA Matar, H Wamidh, B Talib, S Mohammad Mustafa, S Mohammad, et al. (2013) Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3, 30-diaminodipropylamine. Arabian Journal of Chemistry 8(6): 850-857.
- Abdur Rauf, Afzal Shah, Khurram Shahzad Munawar, Abdul AzizKhan, Rashda Abbasi (2017) Synthesis, spectroscopic characterization, DFT optimization and biological activities of Schiff bases and their metal (II) complexes. Journal of molecular structure 1145: 132-140.
- Muhammad Shabbir, Zareen Akhter, Hammad Ismail, Bushra Mirza (2017) Synthetic bioactive novel ether based Schiff bases and their copper (II) complexes. Journal of molecular structure 1146: 57-61.
- AbdurRauf, Afzal Shah, KS Munawar, SaqibAli, M Nawaz Tahir, et al. (2016) Synthesis, physicochemical elucidation, biological screening and molecular docking studies of a Schiff base and its metal (II) complexes. Journal of photochemistry and photobiology 164: 65-72.
- Marimuthu Sakthi, Andy Ramu (2017) Structure, DNA/BSA binding and antibacterial studies of NNO tridentate Schiff base metal complexes. Journal of molecular structure 1149: 727-735.
- Elham SA, Waleed Ahmed El-Said (2014) Synthesis of copper/nickel nanoparticles using newly synthesized Schiff-base metals complexes and their cytotoxicity/catalytic activities. Bioorg Chem 57: 5-12.
- Mirela Feranda zaltlov, Marla Cazacu, Serglu shova, Mlhael Balan, NIcoleta Vornlcu, et al. (2015) synthesis, characterization and antimicrobial activity of new Cu(II) and Zn(II) Complexes with Schiff bases derived from trimethylsilyl-propyl-p-aminobenzoate. Polyhedron 100: 121-131.
- Mostafa Y Nassar, Hisham M Aly, Ehab A Abdelrahman, Moustafa E Moustafa (2017) Synthesis, characterization, and biological activity of some novel Schiff bases and their Co(II) and Ni(II) complexes: A new route for Co3O4 and NiO nanoparticles for photocatalytic degradation of methylene blue dye. Journal of molecular structure 1143: 462-471.
- Arafa AMB, MA Zayed, M El-Desawy, MAH Rakha (2015) Structure investigation of three hydrazones Schiff's bases by spectroscopic, thermal and molecular orbital calculations and their biological activities. Mol Biomol Spectrosc 138: 49-57.
- Elzbieta LC, Marzena S, Anna S, Anna B, Eugenio G, et al. (2015) Chelating ability and biological activity of hesperetin Schiff base. Journal of Inorganic Biochemistry 143: 34-47.
- Jaganathan V, Mahalingam S, Venugopal T, Govindasamy M (2017) Thermal decomposition and kinetic analyses of sulfonamide Schiff's bases in oxygen atmosphere-A comparative study. Chemical Data Collections 9-10: 229-243.
- E Halevas, CM Nday, E Kaprara, V Psycharis, CP Raptopoulou, et al. (2015) Sol-gel encapsulation of binary Zn(II) compounds in silica nanoparticles. Structure-activity correlations in hybrid materials targeting Zn(II) antibacterial use. Journal of inorganic biochemistry 151: 150-163.
- M Abdallah, MA Zayed, Gehad GM (2010) Synthesis and spectroscopic characterization of new tetradentate Schiff base and its coordination compounds of NOON donor atoms and their antibacterial and antifungal activity. Arabian Journal of Chemistry 3(2): 103-113.
- Periyasamy R, Loganathan C, Iruthayaraj A, Poomani K, Thayumanavan P (2017) New amino acid-Schiff base derived from s-allyl cysteine and methionine alleviates carbon tetrachloride-induced liver dysfunction. Biochimie 138: 70-81.
- Muhammad Sirajuddin, Noor Uddin, Saqib Ali, Muhammad Nawaz Tahir (2013) Potential bioactive Schiff base compounds: Synthesis, characterization, X-ray structures, biological screenings and interaction with Salmon sperm DNA. Mol Biomol Spectrosc 116: 111121.
- Mariangela Ceruso, Fabrizio Carta, M Osman, Zeid Alothman, Simona Maria Monti, et al. (2015) Inhibition studies of bacterial, fungal and protozoan p-class carbonic anhydrases with Schiff bases incorporating sulfonamide moieties. Bioorganic & Medicinal Chemistry 23(15): 4181-4187.
- OM Ali, SM El-Medani, DA Ahmed, DA Nassar (2014) Metal carbonyl complexes with Schiff bases derived from 2-pyridinecarboxaldehyde: Syntheses, spectral, catalytic activity and antimicrobial activity studies. J Mol Structure 1074: 713-722.
- OM Ali, SM El-Medani, DA Ahmed, DA Nassar (2010) Preparation, characterization and biological activity of novel metal-NNNN donor Schiff base complexes. Molecular and biomolecular spectrascopy 75(2): 678-685.
- Abbas Ali Salih Al-Hamdani, Abdel Majid Balkhi, A Falah (2016) Synthesis and investigation of thermal properties of vanadyl complexes with azo-containing Schiff-base dye. Journal of Saudi Chemical Society 20(5): 487-501.
- Mozaffar Asadi, Zahra Asadi, Somaye Barzegar Sadi, Leila Zarei, Fatemeh Moosavi Baigi, et al. (2014) Synthesis, characterization and the interaction of some new water-soluble metal Schiff base complexes with human serum albumin. Mol Biomol Spectrosc 122: 118-129.
- S Praveen Kumar, R Suresh, K Giribabu, R Manigandan, S Munusamy, et al. (2015) Synthesis and characterization of chromium(III) Schiff base complexes: Antimicrobial activity and its electrocatalytic sensing ability of catechol. Mol Biomol Spectrosc 139: 431-441.
- Nuriye Kocak, Mustafa Sahin, Semahat Kucukkolbasi, Zehra Ozden Erdogan (2015) Synthesis and characterization of novel nano-chitosan Schiff base and use of lead (II) sensor. Mol Biomol Spectrosc 139(15): 431-441.
- Jin-Qi Xie, Chuan-Hua Li, Jia-Xin Dong, Wei Qu, Lan Pan, et al. (2014) The standard molar enthalpy of formation of a new copper(II) Schiff-base complex and its interaction with bovine serum albumin. Thermochimica acta 598: 7-15.
- Apoorva Upadhyay, Shefali Vaidya, VS Venkatasai, Prabha Jayapal, K Srivastava, et al. (2013) Synthesis and characterization of 3d and 4f metal complexes of Schiff base ligands. Polyhedran 66: 87-96.
- GG Mohamed, MM Omar, A Ibrahim (2009) Nickel complexes of Schiff bases derived from mono/diketone with anthranilic acid: Synthesis, characterization and microbial evaluation. European Journal of Medicinal Chemistry 44(12): 4801-4812.
- Jahangir M, Amit KM, Goutam KP (2018) Highly selective hydrazone based reversible colorimetric chemosensors for expeditious detection of CN- in aqueous media. Inorganica Chimica Acta 474: 22-29.
- Mehmet Aslantas, Engin Kendi, Necmettin Demir, AliE sabik, Mehmet Tumer, et al. (2017) Synthesis, spectroscopic, structural characterization, electrochemical and antimicrobial activity studies of the Schiff base ligand and its transition metal complexes. Microbial Pathogenesis 110: 414-425.
- GG Mohamed, MM Omar, AA Ibrahim (2012) Biological activity studies on metal complexes of novel tridentates Schiff base ligand. Spectroscopic and thermal characterization. European Journal of Medicinal Chemistry 44 (12): 4801-4812.
- M Kalanithi, M Rajarajan, P Tharmaraj, CD Sheela (2012) Spectral, biological screening of metal chelates of chalcone based Schiff bases of N-(3-aminopropyl) imidazole. Mol Biomol Spectrosc 87: 155-162.






























