Role of Chirality in Drugs
Reshma Jayakumar1, Ragavi Vadivel1 and Nallamuthu Ananthi2*
1PSGR Krishnammal College for Women, India
2Department of Chemistry, Karunya Institute of Technology and Sciences, India
Submission: February 6, 2018; Published: February 23, 2018
*Corresponding author: Nallamuthu Ananthi, Department of Chemistry, Karunya Institute of Technology and Sciences, Coimbatore, Tamil Nadu, India, Tel: 99941 49762, Email: ananthi@karunya.edu
How to cite this article: Reshma Jayakumar, Ragavi Vadivel, Nallamuthu Ananthi. Role of Chirality in Drugs. Organic & Medicinal Chem IJ. 2018; 5(3): 555661. DOI: 10.19080/OMCIJ.2018.05.555661
Abstract
About more than half of the drugs currently in use are chiral compounds. Although few drugs were used as racemates, most of the drugs are founds as single enantiomer. With the same chemical structure, most of the enantiomers of chiral drugs exhibit marked differences in biological activities such as pharmacology, toxicology, pharmacokinetics, metabolism etc. In this review we comprised some of the notable chiral drugs, their mechanism of action and their structure activity relationship.
Keywords: Chirality; Chiral drugs; Pharmaceutical Drugs; Pharmacological Activity
Introduction
Chirality has become a major role for the synthesis and development of drugs. Most of the drugs discovered are chiral. The pharmacological activity of drugs depends mainly on its interaction with biological targets such as proteins, nucleic acids and bio membranes. One enantiomer of a chiral drug may be a medicine for particular disease whereas; another enantiomer of the molecule may be not only inactive but can also be toxic. Hence Chirality plays an essential role in drugs. Synthesising compound as single enantiomer is crucial in the design and synthesis of drugs. This review outlines the basic concept of Chirality, the effect of Chirality on the efficiency of drugs and interesting potential chiral drug molecules. History of Drugs During past 120 years there has been a revolution in therapeutics. Medicines have been used to cure disease and relieve pain. With these there is an increase in the abuse of some medicine. So, the law had developed to control the misuse of some medicine. In 1898, Heroin was first introduced. And in later years Aspirin, Opium, Morphine was introduced. And Opium, Morphine, Cocaine in many patent medicines leads to addiction and death. Mrs. Winslow's soothing syrup kills many children due to over dosage of morphine.
Stereoselectivity on Chiral Molecule
Stereoselectivity is observed in drug deposition particularly for those processes which depend on an interaction with a chiral biological macromolecule e.g, active transport process, binding to plasma proteins and drug metabolism. However, if a chiral drug molecule is a substrate for an active transport process then differences between both enantiomer and diasteromer would be expected with preferential absorption of the stereoisomer with a spatial arrangement similar to that of the natural substrate. In metabolism, a process resulting from a direct interaction between a drug and a chiral macromolecule, stereo differentiation is the rule rather than the exception and stereo selective metabolism is probably responsible for the majority of the difference is observed in enantioselective drug deposition. As a result of pharmacokinetics profiles of the enantiomer of drug administered as a racemate may differ.
The Role of Glucuronide
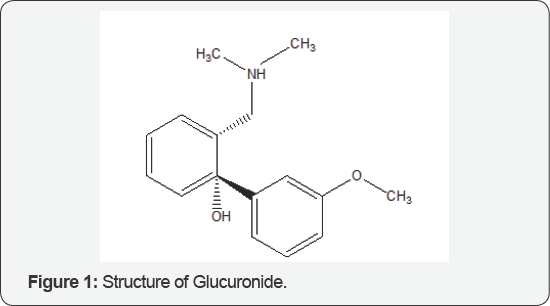
Glucuronide (Figure 1) is a chiral compound found in the hair. Glucuronide can exist as a linear or as cyclic hemiacetal. By the Fischer convection glucuronide has two stereoisomers, D and L Glucuronide, depending on the configuration of C-5. Due to ring closure it has an asymmetric carbon at C-1, resulting in two more stereoisomers named anomers. Depending on the configuration at C-1, there are two anomers of glucuronide, α- and β-form. In β-D-glucuronide the C-1 hydroxy group is on the same side of the pyranose ring as the carboxyl group. In the free sugar acid, the β-form is prevalent, whereas in the organism, the a-form is predominant [1].
The Role of Tramadol
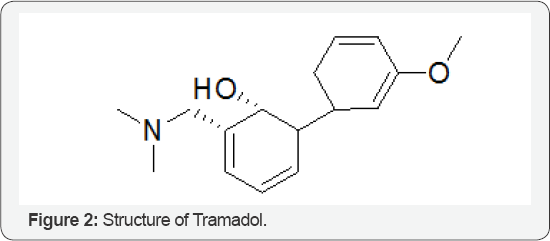
By using high-performance liquid chromatographic assay for the quantitative determination of the analgesic tramadol (Figure 2) act as an active metabolite. Ketamine was used as internal standard. The limit of quantitation of each enantiomer oftramadol and its active metabolite by this method was 0.5 ng/mL; Only 0.5 ml of the plasma sample was required for the determination. The calibration curve was linear from 0.5 to 750 ng/mL for tramadol enantiomers, and from 0.5 to 500 ng/mL for O- dimethyl tramadol enantiomer. Intra and inter day precision [coefficient of variation (CV)] did not exceed 10%. Mean recoveries of 95.95 and 97.87% for (+) R, R- and (-) S, S-tramadol and 97.70 and 98.79% for (+) R, R- and (-)S, S-O- dimethyl tramadol with CVs < 2.15% were obtained. A tramadol is a narcotic pain reliver. The extended release from the tramadol is around for the clock treatment of pain.we should not take tramandol in a severe breathing problem,or blockage in your stomach or intestine,or if you have recently used alcohols,sedatives [2].
The Role of Glutathione
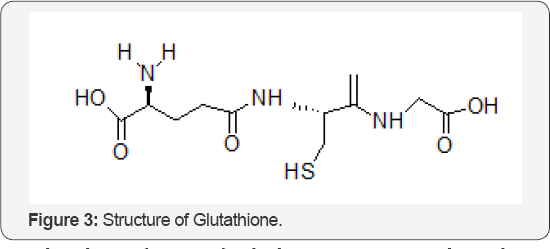
Glutathione (Figure 3) which serve as intermediate cluster carriers in iron-sulphur cluster trafficking. The [2Fe-2S]-bound halo forms of glutathione proteins display cysteinyl coordination from exogenous glutathione, in addition to contact from protein- derived Cysteinyl. Those mechanistic studies that investigate the role of exogenous glutathione in defining cluster chirality, ligand exchange, and the cluster transfer chemistry of Saccharomyces cerevisiae. Systematic perturbations were introduced to the glutathione-binding site by substitution of glutathione molecule.It revealed that electrostatic contacts are of key importance for positioning the exogenous glutathione that in turn influences the chiral environment of the cluster. All glutathione derivatives were reconstituted by standard chemical reconstitution protocols and found to transfer cluster to apo ferredoxin at rates comparable to native protein. Kinetic analysis of cluster transfer from halo derivatives to apo Ferridoxin has led to a mechanistic model for cluster transfer chemistry of native halo Glutathione, and identification of the likely rate-limiting step for the reaction. It is a Xenobiotic compound used for metabolism in the intestine. It is a compound synthesized from cysteine, a most Important method of disposal of waste from the body It is very important for the formation of white and red blood cells throughout the immune system. Glutathione's clinical uses include the prevention of oxygen toxicity in hyperbaric oxygen therapy, treatment of lead and other heavy metal poisoning, lowering of the toxicity of chemotherapy and radiation in cancer treatments, and reversal of cataracts [3].
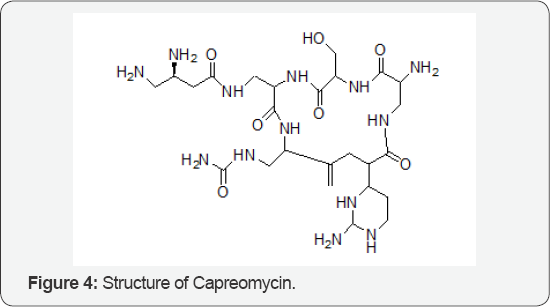
The Role of Capreomycin
The mechanisms Chiral Selector Chiral recognition mechanism, Examples, separated enantiomers and Chiral recognition is based on inclusion of the bulky hydrophobic group of the analyte into the hydrophobic cavity and on lateral interactions of the hydroxyl groups, such as hydrogen bonds and dipole-dipole interactions, with the analyte. The drug used in the treatment of tuberculosis is Capreomycin (Figure 4). It was discovered in 1960.It is an antibiotic used in the treatment for TB. It is given by injection through veins or muscles. Common side effect includes kidney problem, hearing problem, poor balance at the site of injection. Other side effect includes paralysis, resulting to inability in breath. It is not recommended with streptomycin or other medications which may damage the auditory vestibular nerve. It is not recommended during pregnancy as it may cause kidney or hearing problem to a baby [4].
The Role of Cytarbine
There is a huge interest in devising analytical methods for the analysis and separation of enantiomers, thus leading to improved treatments of marketed pharmaceutical products. Cytarabine is a nucleoside analogue used as a chemotherapeutic agent for the treatment of acute lymphoblastic and myelogenous leukaemia. It is used to separate the enantiomers of anticancer reagent cytarabine by HPLC and investigate the effects of cytarabine enantiomers on specific cancer cell lines in order to identify if the enantiomers possess any specificity. Leukaemia is not a single disease, which is related to number of cancer cells that's starts in the blood forming cells of bone marrow. Chemotherapy is a major form, used to cure leukaemia. The drug which is used for the treatment is cytarbine. The cytarbine is also known as cytosine arabinosine, is a chemotherapeutic medication to treat acute myeloid leukaemia it is given by injection through vein, under the skin or through cerebrospinal fluid. Common side effect includes bone marrow suppression, vomiting, diarrhea, liver problem, rash, ulcer formation in mouth and bleeding. other serious effect includes loss of consciousness, lung disease, allergic reaction. Use in pregnancy may harm the baby. It was approved on 1969 by WHO [5].
The Role of Cyclodextrin

The use of cyclodextrins (Figure 5) as chiral selectors in capillary electrophoresis for the enantio separation of drugs. The use of dual chiral systems, modified capillaries, non-aqueous media or microfluidic devices is also included and the mechanisms for enantioseparation of drugs and the inversion of the enantiomer migration order are formed. The most relevant applications developed to carry out the quantitation of chiral drugs, to assess the enantiomeric purity of pharmaceutical formulations, to study their metabolism or to achieve criminalistic or forensic investigations are described. Cyclodextrin is an oligosaccharide, having outer hydrophilic and centre hydrophobic cavity. It is used as an eye drop solution for the disease of opthalmics [6].
The Role of Flutamide
The drug delivery performance of the functionalized single-walled carbon nanotube with a carboxylic acid group for anticancer drug in the gas phase as well as water solution by means of density functional theory calculations. It confirmed the energetic stability of the optimized geometries and revealed that the nature of drug adsorption on the functionalized carbon nanotube is physical. It showed that the hydrogen bonding between active sites of Flutamide (Figure 6) molecule and the carboxyl functional group of the nanotube plays a vital role in the stabilization of the considered configurations. The natural bond orbital analysis suggested that the functionalized nanotube plays the role of an electron donor and Flutamide molecule acts as an electron acceptor at the investigated complexes. In addition, molecular dynamics simulation is also utilized to investigate the effect of functionalized carbon nanotube chirality on the dynamic process of drug molecule adsorption on the nanotube surface. The results demonstrated that drug molecules are strongly adsorbed on the functionalized nanotube surface with chirality, as reflected by the most negative vander Waals interaction energy and a high number of hydrogen bonds between the functionalized nanotube and drug molecules.the role of flutamide is used for the patient with prostatic intraepithelial neoplasia [7].

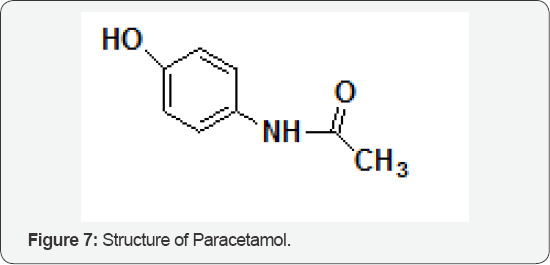
The Role of Paracetamol
The interactions of cystamine, N-acetylcysteine, 2-mercaptopropionylglycine and methionine with N-acetyl 4-aminophenol (paracetamol) (Figure 7) have been examined during its metabolism to a covalently bound product in a vitro mouse hepatic microsomal system. Of the compounds used only methionine failed to reduce the amount of covalently bound paracetamol-derived radioactivity. These results indicate that the effectiveness of methionine in reducing paracetamol hepatotoxicity in vitro is achieved by mechanisms other than those involving direct interactions with the hepatic mixed- function oxidase system or its oxidation products. In contrast, cysteamine, N-acetylcysteine and 2-mercaptopropionylglycine can directly inhibit the binding of paracetamol-derived radioactivity in vitro, and may do so by processes which affect both the formation and the subsequent binding of a reactive paracetamol metabolite [8].
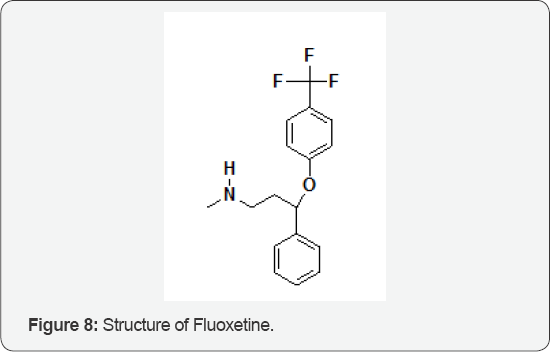
The Role of Fluoxetine
The Role of FluoxetineFluoxetine (Figure 8) is an antidepressant; a selective serotonin reuptake inhibitor (SSRI) used primarily in the treatment of major depression, panic disorder and obsessive- compulsive disorder. Chiral separation of racemic fluoxetine is necessary due to its enantioselective metabolism. In order to develop a suitable method for chiral separation of fluoxetine, cyclodextrin (CD) modified capillary electrophoresis (CE) was employed. A large number of native and derivatized, neutral and ionized CD derivatives were screened to find the optimal chiral selector. As a result of this process, hepatis (2,3,6-tri-O-methyl)- P-CD (TRIMEB) was selected for enantiomeric discrimination. A factorial analysis study was performed by orthogonal experimental design in which several factors are varied at the same time to optimize the separation method. The optimized method (50 mm phosphate buffer, pH = 5.0, 10 mm TRIMEB, 15 °C, + 20 kV, 50 mbar/1 s, detection at 230 nm) was successful for baseline separation of fluoxetine enantiomers within 5 min. The method was validated according to ICH (The International Conference on Harmonisation) guidelines and proved to be sensitive, linear, accurate and precise for the chiral separation of fluoxetine [9].
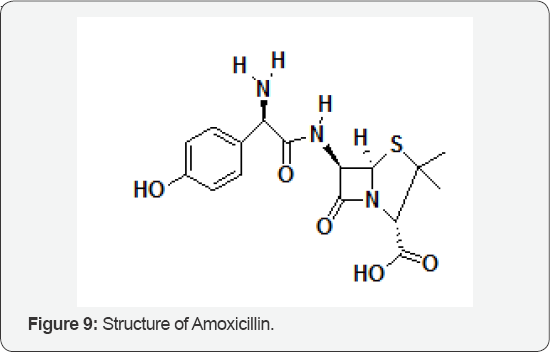
The Role of Amoxicillin
The influence of iron species on amoxicillin (AMX) (Figure 9) degradation, intermediate products generated and toxicity during the photo-Fenton process using a solar simulator were evaluated. The AMX degradation was favored in the presence of the potassium ferrioxalate complex when compared to FeSO4. Total oxidation of AMX in the presence of FeOx was obtained after 5 min, while 15 min were necessary using FeSO4. The results obtained with Daphnia's magna bioassays showed that the toxicity decreased from 65 to 5% after 90 min of irradiation in the presence of FeSO4. However, it increased again to a maximum of 100% after 150 min, what indicates the generation of more toxic intermediates than AMX, reaching 45% after 240 min. However, using FeOx, the inhibition of mobility varied between 100 and 70% during treatment, probably due to the presence of oxalate, which is toxic to the neonates. After 240 min, between 73 and 81% TOC removal was observed. Different pathways of AMX degradation were suggested including the opening of the four-membered β-lactamic ring and further oxidations of the methyl group to aldehyde and/or hydroxylation of the benzoic ring, generating other intermediates after bound cleavage between different atoms and further oxidation to carboxylates such acetate, oxalate and propionate, besides the generation of
The Role of Cocaine
By using a high sensitivity on line preconcentration method, cation-selective exhaustive injection and sweeping MEKC , for the analysis of cocaine, benzoyl cocaine, norcocaine and coca ethylene. They monitored the effects of several of the CSEI sweeping-MEKC parameters including the PH, the concentrations of an organic modifier, the injection length of the high-conductivity buffer and the injection of the sample-to optimize the separation process. The optimal BGE was 100 mm phosphoric acid containing 75 mm SDS with 10% tetrahydron as the organic modifier. In addition electrokinetic injection of the sample at 15 kv for 900 provided both high separation efficiency and the enhanced sweeping sensitivity. under the optimal conditions they had analyzed cocaine in human urine sample prepared using off-line SPE to minimize the can be influence of matrix. Under optimal condition, CSEI- sweeping -MEKC used to determine cocaine and it metabolism with high sensitivity in human urine [11].
The Role of Flecainide
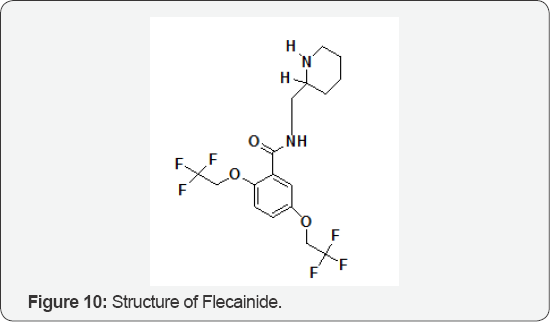
The molecules which possess a chiral center exist in two forms that are indistinguishable in terms of most of their chemical and physical properties. However, when chiral compounds are placed in a chiral environment differences between enantiomers become evident. Stereoselectivity in drug action at specific receptors, with ion drugs, such as flecainide (Figure 10), which do not exhibit stereoselectivity in action on sodium channels in the heart or isolated Purkinje fibers. Enantiomers may exhibit similar, opposing, or different actions, sometimes at the same receptor. For example, the antihypertensive agent labetol is both an α- and β-adrenoceptor antagonist. The drug possesses two chiral centers and is used as a mixture of four diastereoisomers. The R,R form provides most of the (3-blocking activity while the S,R diastereoisomer is a α-receptor antagonist. The S,S and R,S forms do not contribute significantly to the pharmacology of the drug. They discussed about stereoselectivity in drug disposition and metabolism have emerged as important variables in the action or toxicity of some drugs and complicating the interpretation of plasma concentration data in relation to therapeutic effect [12].
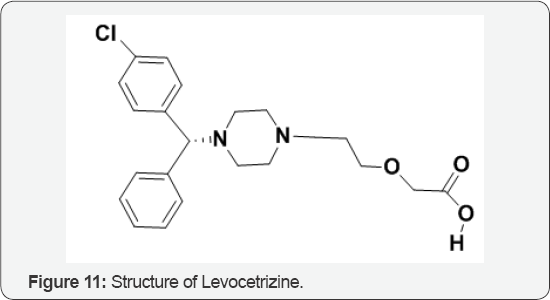
The Role of Levocetrazine
Isomerism finds its importance in the field of clinical pharmacology and pharmacotherapeutics, as isomers differ in their pharmacokinetic and pharmacodyanamic properties. Drug isomerism has opened a new era of drug development. Currently, knowledge of isomerism has helped us in introducing safer and more effective drug alternatives of the newer as well as existing drugs. Many existing drugs have gone chiral switch i.e., switching from racemic mixture to one of its isomers. Cetrizine to levocetrizine (Figure 11) is one of such examples, where effective and safer drug has been made available. In this article, we have attempted to review the basic concepts of stereochemistry and chirality and their significance in pharmacotherapeutics. Various pharmacological aspects such as pharmacokinetic and pharmacodynamic variations resulting out of chirality has been discussed in detail in this article [13].
The Role of Pheniramine
By using solid phase extraction of chiral separation of pheniramine (Figure 12) was achieved on the C18 cartridge and Amy coat and chirelpak chiral columns in human plasma. pheniramine were resolved using n-hexane as a mobile phase. The separation was carried out at 25 C temperatures with the detection at 220 nm for pheniramine the flow of rates of mobile phase were 0.5 ml min. The absolute configurations of the eluted enantiomer of the reported drugs were determined by stimulation studies. It was observed that the order of enantiomer elution of the reported drugs was S-Pheniramine>R-Pheniramine. The mechanism of separation was also determined at the supramolecular level by considering interactions and modelling results. The reported SPE-chiral high performance liquid chromatography methods are suitable for the enantiomeric analyses of these drugs in any biological sample. In addition,stimulation studies may be used to determine the absolute configuration of the first and second eluted enantiomers [14].

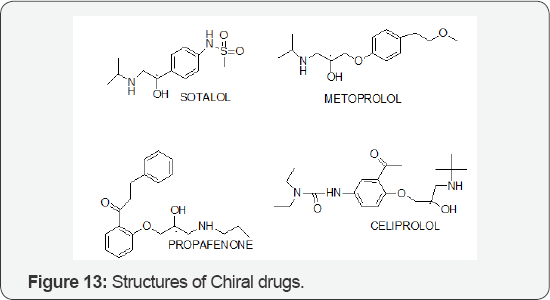
The Role of Solatol, Metoprolol, Propafenone and Celiprolol
This study aimed to determine the Trans epithelial transport characteristics of chiral drug enantiomers across Caco-2 cell monolayers, a model of human intestinal epithelial membrane. Six chiral aryloxy enantiomers (atenolol, sotalol, celiprolol, carvedilol, metoprolol and propafenone) (Figure 13) were tested in bi-directional transport studies. The separation and quantitation of these enantiomers were performed by reversed- phase high-performance liquid chromatography (RP-HPLC) using 2, 3, 4, 6-tetra-O-acetyl-β-D-glucopyranosyl isothiocyanate (GITC) as a pre-column derivatizing agent. Bi-directional transport studies, demonstrated that celiprolol and carvedilol exhibited significant enantioselectivity i n polarized transport at the concentration range tested. The efflux ratio (ER) for (R)-(+)- celiprolol was 8.96, but it was much lower for (S)-(-)-celiprolol which is 3.42 at the concentration of 96.0 μm; carvedilol had the same transport behaviour as celiprolol while the difference between the ER values of two enantiomers was not as significant as celiprolol at the concentration of 5.0 μM. They are 2.41 for (R)-(+)-carvedilol and 1.98 for (S) (-)-carvedilol. But in the transport studies of racemic atenolol, sotalol, metoprolol and propafenone, no enantioselective transport were observed over the concentration range tested. Because P-glycoprotein (P-gp) is highly expressed in Caco-2 cells, we inferred that P-gp might participate in the transport processes of celiprolol and carvedilol in chirally discriminative ways [15].
Conclusion
Chirality is an important consideration in the risk assessment of pharmaceuticals. Chiral separation enantioselective analysis and asymmetric synthesis technology is rapidly improving. Therefore, it is used to test the individual pure enantiomer of chiral compounds to justify the chiral products are manufactured as the racemates.
References
- HyerimYu, Wo Jun Jang, Byoung duckpark, HS Chul, Ho Jeong, et al. (2011) Pigmentation in drug incorporation into hair. Forensic science international journal 63(1-3): 19-29.
- MA Campanerio, Calahorra, M Valle, T Honorato (2010) Enantiomeric separation of tramadol and its active metabolite in human plasma by chiral high performance liquid chromatography. Journal of chromatography B 878(3-4): 481-486.
- Chen H, J Chen, J Guo, Y Wen, J Liu, et al. (2012) Evaluation of glutathione redox cycle in chiral perturbation approach. Aquatic toxicology 84(1): 120-121.
- BB Plikaytis, TM Shinnick (2005) Antimicrobial agents and chemotherapy molecule analysis of capreomycin in mycobacterium tuberculosis. International journal of infectious disease 49(4): 31923197.
- WH Brooks, WC Guinda, KG Daniel (2011) The significance of chirality in drug design and development. Curr Top Med Chem 11(7): 760-770.
- JM Saz, MLMarina (2016) Advantages on the use of cyclodextrins in the chiral analysis of drugs by capillary electrophoresis. Journal of chromatography 1467(2): 79-94.
- M kamel, Heider raissi, Alimorsali (2018) Assessment of the adsorpation mechanism of flutamide anticancer drug on the functionalized single walled carbon nanotube surface as a drug delivary vehicle: An alternative theoretical approach based on DFT and MD. Applied surface science 434: 492-503.
- JM Tredger, HM Smith, R Williams (1980) Effects of sulphur containing compounds an activation and covalent binding in a mouse hepatic microsomal system. Toxicol Lett 5(5): 339-344.
- DM Budau Gabriel, Hancu, Laszlo Gagyi, AuraRusu, Hajnal Kelemen (2017) Enantioselective analysis of fluoxetine in pharmaceutical formulations by capillary zone electrophoresis. Saudi Pharm J 25(3): 397-403.
- AG Trovo, F Raquel, Nogueria, AA Amadeo, R Fernandez, et al. (2011) Degradation of the antibiotic amoxicillin by photo-fenton pro cess- chemical and toxicological assessment. Water research 45(3): 13941402.
- Hsiu Li Su, Lan-lug Feng, Hsiu-Ping Jen, You-Zung Hsieh (2008) Determination of cocaine and its metabolitics using cation-selective exhaustive injection and sweeping -MEKC. Electrophorosis 29(20): 4270-4276.
- DS Davis (2013) Chirality in drug design and synthesis, 3-chirality, drug metabolism and action. International J Biomed Sci 379(1): 45-51.
- Naveen Chabra, ML Asari, Deepak Padmanbhan (2013) A review of drug isomerism and its significance. Int J Appl Basic Med Res 3(1): 1618.
- Imran Ali, ZA Abdulrahman, Al- Warthan, SD Alam, JA Frooqi (2014) Enantiomeric separation and simulation studies of Pheniramine, Oxybutynin, and Brinzolamide chiral drugs and Amylose based columns. Chirality 26(3): 136-143.
- Yen Tian, Yinge He, Haihong, Hul LuWang, Su Zeng Acta (2012) Determination of the enantioselectivity of six chiral aryloxy aminopropanol drug transport across caco -2 cell monolayers. Pharmaceutica sinica 58(3): 168-173.






























