Synthesis, Characterization, Thermodynamic and Bi-ological Activity Studies for New Complexes of Some m Transition Metals and Zinc With Schiff's Bases [2,5di (1-Thi-Ophen-2-yl-Ethylidene)-Hydrazine) 1, 3, 4-Thia- diazole]
Hamid Eissa*, Naser Shaalan and Shatha Mahde
Assistant Professor in Physical Organic Chemistry - Chemistry Department, Applied College Sciences, University of Hajah, Yemen
Submission: January 30, 2018; Published: February 12, 2018
*Corresponding author: Hamid Eissa, Department of Chemistry, College of Science for Women, Baghdad University, Baghdad, Iraq, Email: hamedesia2003@yahoo.com
How to cite this article: Hamid E, Naser S, Shatha M. Synthesis, Characterization, Thermodynamic and Biological Activity Studies for New Complexes of Some Transition Metals and Zinc With Schiff’s Bases [2,5di (1-Thi-Ophen-2-yl-Ethylidene)-Hydrazine) 1, 3, 4-Thiadiazole]. Organic & Medicinal Chem IJ. 2018; 5(2): 555659. DOI: 10.19080/OMCIJ.2018.05.555659
Abstract
New metal complexes of type [M (L)] Cl2, [M (L) Cl2] [(M = Co (II), Ni (II), Cu (II) and Zn (II)] were prepared using the ligand (L) =[2,5di (1-thiophen-2-yl-ethylidene)-hydrazine) 1,3,4-thiadiazole]. The Schiff bases were condensed from [2-acetylthiophene] with [2,5-dihydrazino- 1,3,4- thiadiazole] in alcoholic medium. The Schiff bases were tetra dentate ligand that were used for complexion with Co2+, Ni2+, Cu2+ and Zn2+ ions. The prepared complexes were characterized by FTIR, UV-vis as well as magnetic susceptibility, conductivity measurements, atomic absorption, and thermal analysis & Schiff bases characterized by FTIR, UV-vis, JH-NMR and LC mass spectra and, thermal analysis. The activation thermodynamic parameters, such as AE*, AH*, AS* and AG* are calculated from the TGA curve using Coats-Red fern method. From the spectral measurements, structures for the complexes were proposed. Preliminary in vitro tests for antimicrobial activity show that all prepared compounds display good activity to Staphylococcus aureus, Escherishia coli, Pseudononas aeroginosa andCndida albicans.
Keywords: Schiff Base; 1,3,4-Thiadiazole; Microwave Irradiation; Thermodynamic Parameters; Ultrasonic; Biological Activity
Introduction
Increasing physiological importance of nitrogen donor organic compounds and active role played by coordination certain metal ions to them [1,2]. Have interested use in synthesizing and studying structural aspects of metal complexes with some nitrogen donor ligands [3]. Thus, Schiff base hydrazones are also interesting from the point of view of pharmacology. Hydrazone derivatives are found to possess antimicrobial, antitubercular, anticonvulsant and anti-inflammaty activities [4-7]. Schiff bases- bimolecular condensation products of primary amines with aldehydes- represent valuable intermediates in organic synthesis and, at the same time, compounds with various applicos [8]. Schiff bases resulted from aromatic ketone ortho-substituted group have initially aroused the researches' interest because of their ability to act as bidentate ligands for transition metal ions. Thiophene; thiadiazole and its derivatives form an important class of organic compounds due to their structural chemistry and biological activities as analgesic, antipyretics and antiinflammatory [9]. Even the simplest Thiophene derivatives are widely used analgesic medicines. Thiophene is efficient extract ants of metal ions and they have potential to form different types of coordination compounds. In addition, thiophene can form a variety of Schiff bases and are reported to be superior reagents in biological, clinical and analytical applications [10,11].
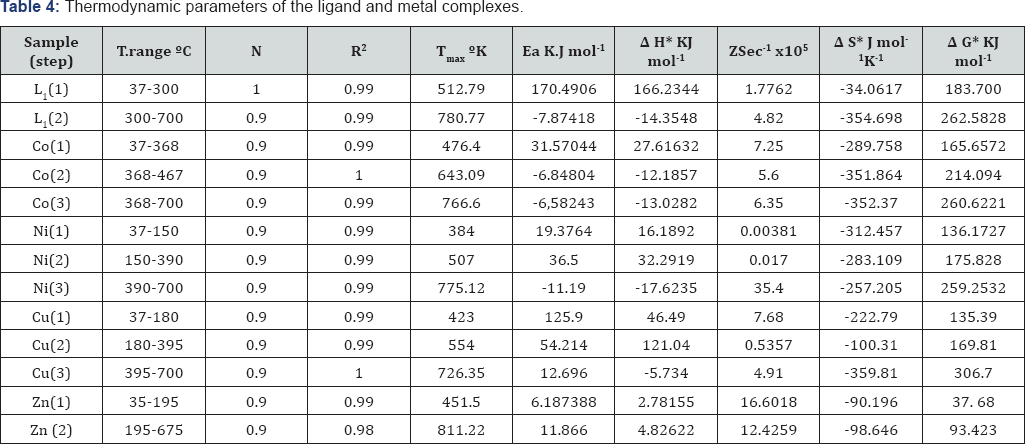
In continuation of our work on the metal complexes of Schiff bases, we report here the study of some new, Co(II), Ni (II) , Cu (II)and Zn (II), complexes of Schiff bases derived from [2-acetylthiophene] with [2,5-dihydrazino-1,3,4-thiadiazole] [12] . Preparation, characterization and antibacterial activity of above metal complexes with this Schiff bases are reported here. From the TGA curves recorded for the successive steps in the decomposition process of these ligand and complexes, it was possible to determine the following characteristic thermal parameters for each reaction step: Initial point temperature of decomposition (Ti): the point at which TG curve starts deviating from its base line. Final point temperature of decomposition (Tf): the point at which TG curve returns to its base line. Peak temperature, i.e. temperature of maximum rate of weight loss: the point obtained from the intersection of tangents to the peak of TG curve. Mass loss at the decomposition step (Dm): it is the amount of mass that extends from the point Ti up to the reaction end point Tf on the TG curve, i.e. the magnitude of the ordinate of a TG curve. The material released at each step of the decomposition is identified by attributing the mass loss (Dm) at a given step to the component of similar weight calculated from the molecular formula of the investigated complexes, comparing that with literatures of relevant compounds considering their temperature. This may assist identifying the mechanism of reaction in the decomposition steps taking place in the complexes under study. Activation energy (E) of the composition step: the integral method used is the Coats-Redfern equation [13]. For reaction order n^1or n= 2, which when linearised for a correctly chosen n yields the activation energy from the slop.
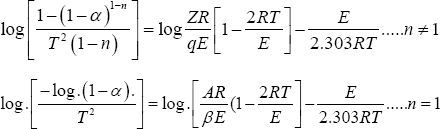
ΔS*= 2.303R [Log(Δh/K Tmax )], ΔH*=E-RTmax' , ΔG*= ΔH* -Tmax ΔS* where: α= fraction of weight loss, T = temperature (°K), n = order of reaction, A or Z = pre-exponential factor, R = molar gas constant, E = activation energy and q = heating rate. Order of reaction (n): it is the one for which a plot of the Coats-Red fern expression gives the best straight line among various trial values of n that are examined relative to that estimated by the Horovitz-Metzger method [14].
Experimental
All chemical used were of reagent grade (supplied by either sigma Aldrich or fluka) and used as supplied. The FTIR spectra in the range (4000-400) cm-1 cut were recorded as CeI disc on FTIR.4200 Jasco Spectrophoto meter. The UV-Visible spectra were measured in ethanol using Shimadzu UV-Vis. 160 A-Ultra-violet Spectrophoto meter in the range (200-1000) nm. Magnetic Susceptibility measurements for complexes were obtained at room temperature using (Magnetic Susceptibility Balance) Jhonson Mattey Catalytic Systems Division. Gall encamp M.F.B600.010 F melting point apparatus were used to measure the melting point of all the prepared compounds. Elemental microanalysis was carried out using CHNOS Elemental Analyzer Model 5500 Carlo-Erba Instruments (Italy).
1- Synthesis of [2,5-dimercapto-1,3,4-thiadiazole] [A]
A mixture of (99%) hydrazine hydrates (5 mL, 0.02 mol) and carbon disulfide (15 mL, 0.02 mol) with dry pyridine (50 mL) was refluxed for (5 h). Then the excess solvent was then distilled off by rotary evaporator, and the resulting solid was separated out by adding (25 mL) of water and (5 mL) of hydrochloric acid. The mixture was then filtered and the solid was recrystalized from ethanol.
2-Synthesis of [2,5-dihydrazino-1,3,4-thiadiazole] [B]
To 2,5-dimercapto-1,3,4-thiadiazole [A] (1.5 g,0.01 mol) dissolved in ethanol, hydrazine hydrate (5 mL,0.02 mol) was added dropwise with stirring and the mixture was then refluxed for (6 h), then the excess solvent was distilled off by rotary evaporator. Filtered the resulting solid which was separated out on cooling and recrystalized from ethanol to give the desired product. Mp, yield, CHNS analysis in Table 1.
3-[2,5di (1-thiophen-2-yl-ethylidene)-hydrazine) 1,3,4-thiadiazole] [L]
Method 1: A mixture of [B] (1.46 g, 0.01 mol) in absolute ethanol (50 mL) with 2-acetylthiophene (1.90 g, 0.02 mol) in absolute ethanol (50 mL) in (250ml) round bottom flask and add (5) drops of glacial acetic acid was refluxed on water bath at (80 C) for (8 h). The reaction mixture was then allowed to cool at room temperature, and the precipitate was filtered dried, and recrystallized from ethanol to give yellow powder.
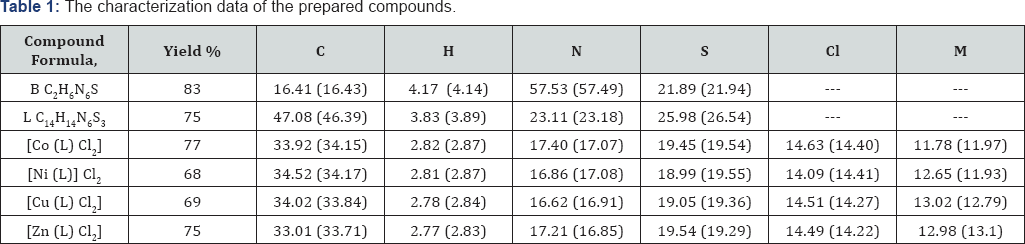
Method 2: A mixture of [B] (1.46 g, 0.01 mol) with (1.90 g, 0.02 mol) 2-acetylthiophene were ground with a mortar, mixed, dried and subjected to microwave irradiation 280W for (10) minutes, after completion the reaction mixture was cooled to room temperature the solid obtained was recrystallized twice from absolute ethanol, some of physical data for these four compounds are listed in Table 1.
Preparation of complexes
Method 1: An ethanol solution of the metal salt of Co(II), Ni (II), Cu (II) and Zn (II) were added to an ethanolic solution of (L) in 1:1 (metal : ligand) molar ratios. After stirring for 2 hours with heating 500C, crystalline colored precipitates formed cooling at room temperature, the resulting solids were filtered off, washed with distilled water, dried and recrystallized from ethanol and dried at 500C.
Method 2: An ethanol solution of the metal salte of Co(II), Ni (II), Cu (II) and Zn (II)were added to an ethanolic solution of (L) in 1:1 (metal : ligand) molar ratios. And put in ultrasonic bath heating 500C After 30 mints crystalline colored precipitates formed, cooling at room temperature, the resulting solids were filtered off, washed with distilled water, dried and recrystallized from ethanol and dried at 500C. yield, C.H.N.S analysis in Table 1.
Results and Discussion
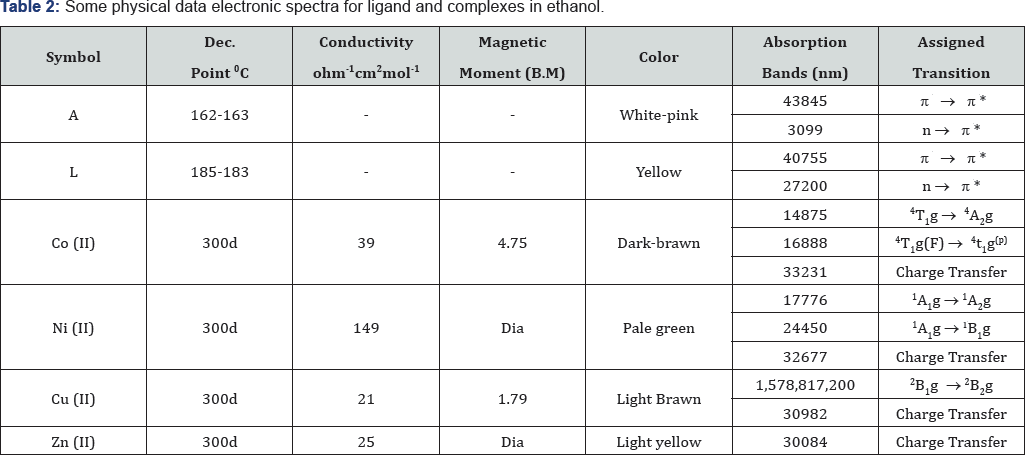
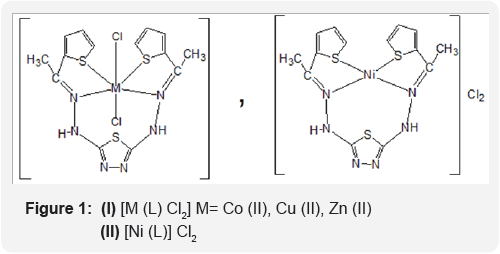
The synthetic procedure of Schiff base ligand is presented in Scheme 1. The reactions of divalent transition metal ions viz., Co (II), Ni (II), Cu (II) and Zn (II). The composition of the complexes formed in solution has been established by mole ratio and job methods. In both cases the results reveals (1:1) metal to ligand ratio yielded the corresponding metal chelates. The decomposition point, color and electronic absorption bands for ligand and complexes are shown in Table 2. The bands are classified into three distinct groups: The intermolecular transitions appear in the UV region, charge transfer from ligand to metal, and d-d transitions appear in the UV-Visible region.
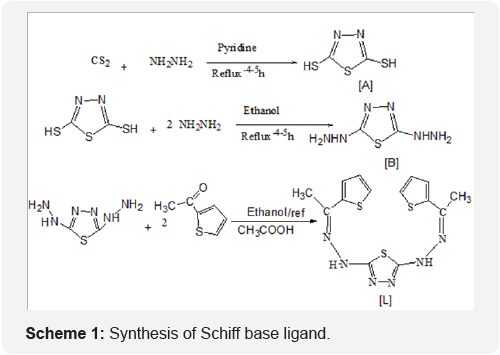
[2,5di (2-thiophene methyl hydrazone) 1,3,4-thiadiazole] [L]
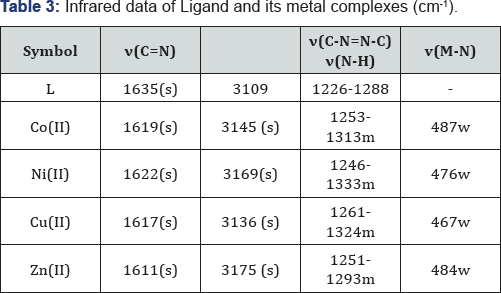
The FT-IR spectra show the disappearance of the two absorption bands due to (-NH2) stretching of hydrazone thiadiazole [L] showed all the suggested bonds for olefinic (CH), (C=C) aromatic, endocyclic (C=N) and exocyclic imine group. Stretching vibrations in addition to out of plane bending of substituted aromatic ring. The prepared compounds (Schiff base) exhibited the stretching band near the region (12131253) cm-1 this due to (=N-N=) cyclic group; a band at 3109 cm-1 attributed to NH stretching (NH), 1635cm-1 (u C=N Stretching of amine), (1226-1288) cm-1 (Characteristic bands of thiazole ring). All the spectral data for other compounds are listed in Table 3.
1H-NMR spectrum of compounds [L], shows the following characteristic chemical shift, (CDCL3-d6) ppm. The sex proton for tow thiophene ring appeared at (5 6.531 - 7.495) ppm, the signal at (5 8.98) attributed to (N=C-H) tow proton (azomethine), the signal at (5 9.755) ppm, attributed to (N-H) proton, 5= 2.43.4 (5 s, 6H, CH3) 5=1. 44 - 1.744 (Solvent+H2O). The positive ion mass spectral analysis of (L) observe at m/z 362.98 (M+1), confirms the theoretical molecular weight i.e. 362.50.
Infrared spectral analysis of metal complexes
The infrared spectra of the ligands shows (uN-H) at 3109 cm-1. A shift of this band in all the metal complexes indicates the removal of proton NH group of thiophene ring during the chelation. The sharp intense band at 1635 cm-1 in the ligands can be assigned to uC=N (azomethine). A shift (Au = 13-24 cm-1) in uC=N (azomethine) is observed upon coordination indicating that the nitrogen of azomethine group is involved in coordination. The entire complexes show broad band in the region (3285-3378) cm-1 which may be assigned to (u O-H) of coordinated water to account for the octahedral stereochemistry of the metal complexes [15].
The bands at 514cm'1 in Co (II) complexes, 591 cm-1 in Ni(II) complex and 590cm-1 in Cu(II) complexe may be due to metal- nitrogen stretching vibration [16,17]. All the metal complex involved in coordination In the free ligand, the band at 1606 cm-1 is assigned to the stretching of C=N(thiazole ring) .On complexion, this band was shifted to a lower frequency region. This shift is probably due to the lowering of bond order of the carbon-nitrogen bond resulted from complexion of the metal to the ligand through nitrogen in (uC=N) compared to its respective ligands. This suggests that the nitrogen atom of the ring has not participated in the chelation. However, in water containing chelates, this band is observed as a broad with structure this may be due to coupling of the bending mode of coordinated [18].
Thermal Analysis
To understand thermal decomposition process, Schiff and its metal complexes were examined by thermo gravimetric analysis in the temperature range of 35-700°C. The obtained thermo analytical results from TGA curves for all these compounds are given in Table 4. The decomposition was complete at 693 °C for all the complexes. The data from the thermo gravimetric analyses indicated that the decomposition of the complexes and the ligand proceeds in (two-four) steps. The final decomposition products were metal oxide mixture formed above 598 °C for the metal [19].
Biological Activity
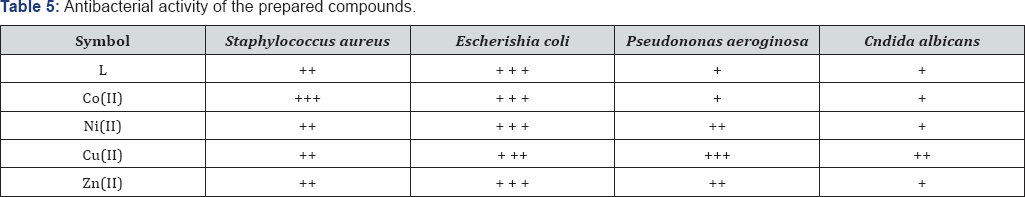
Note: (-) = no inhibition, (+) = (5-10) mm, (++) = (11-20) mm, (+++) = more than (20) mm.
With a view to explore the possibility of obtaining thiophene ring system, such biological activity prompt us to biologically useful complexes that contain 1,3,4- thiadizole and prepare some new series containing the above mentioned unite. The antimicrobial activity of these compounds was determined by the ager diffusion method used which was Staphylococcus aureus, Escherishia coli, Pseudononas aeroginosa and Cndida albicans [18]. In this method a standard 5mm diameter sterilized filter paper disc impregnated with the compound (1 mg per 1 ml of acetone) was placed on an agar plate seeded with the test organism. The plates were incubated for 24 hours at 370C. The zone of inhibition formed was measured in mm and are represented by (+), (++) and (+++) depending upon the diameter and clarity, Table 5. The preliminary screening result reveal that compound contained thiadizole and thiophene complexes exhibits highest antibacterial activity against Escherishia coli (Figure 1).
References
- Naser S, Samh H, Mohamad C (2014) Synthesis, Characterization And Biological Activity Of Metal Complexes With Schiff Bases Derived From [4-Antipyrincarboxaldehyde] With [2-Amino-5-(2-Hydroxy-Phenyl)-1,3,4- Thiadiazole]. Damascus University J BASIC SCIENCES 30(2): 83102.
- Anna C, Crstina L, Antonino R, Roberto R, Paolo V, et al. (2007) A Predictive Model for Aggressive Non-Hodgkin's Lymphoma. Molecules 12: 1482-1495.
- Bougrin K, Loupy A (2005) Microwave assisted solvent free heterocyclic synthesis J Photochem & Photobiol Photochem 6(2-3): 139-167.
- Vicini P, Zani F, Cozzini P, Doytchinova I (2002) Hydrazones of 1, 2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 37: 553-564.
- Kocyigit-Kaymakcioglu B, Rollas S, Farmaco (2002) 57: 595.
- Ragavendran JV, Sriram D, Patel SK, Reddy IV, Bharathwajan N, et al. (2007) Design and synthesis of anticonvulsants from a combinedphthalimide-GABA-anilide and hydrazone pharmacophore. Eur J Med Chem 42(2): 146-151.
- Rollas S, Gulerman N, Erdeniz H (2002) Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and3- acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Farmaco 57(2): 171174.
- Nazk MA, Naser DS, Sahar SH (2015) Synthesis, Spectroscopic, Thermodynamic and Biological Activity Studies of Schiff Base and Metal Complexes Derived from 2-[1H-Pyrrol-2-Ylimino Methyl]- 5-Phenyl-1,3,4-Oxadiazo le. Global J Science Frontier Res 15(3).
- Abbiati G, Arcadi A, Attanasi OA, De Crescentini L, Rossi E (2001) Synthesis of functionalised pyrazolones and imidazolines /imidazoles through divergent cyclisation reactions. Tetrahedron 57(10): 20312038.
- Yang ZY, Yang RD, Li FS and Yu KB (2001) (Z)-4-{1-[(2-Hydroxyethyl) amino]ethylidene}-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one. Polyhedron 19: 2599.
- Joshi KT, Dabhi HR, Pancholi AM, Rana AK (1996) Ligand field parameters of Cu (II), Ni (II) and Co (II) chelates with some4- substituted-1-phenyl-3-methyl-2-pyrazolin-5-ones. Orient J Chem 12(3): 287-290.
- Naser Shaalan, Yasmine Hussein (2018) Preparation, Spectral Characterization, Structural study, and Evaluation of antibacterial activity of Metal Complexes with Schiff base derived from (N, N-Bis (1,5-dimethyl-2-phenyl-1,2-dihydro- pyrazolidine-3-one)-1,2-diamino ethane) Rese. J Pharm Bio and Chem Sci 9(1): 376-385.
- Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric. Nature 201: 68-69.
- Horovitz HH, Metzger G (1963) Analysis of thermogravimetric traces as an approach to determine the mechanism of dissociation of copper (Il)-a-aryl azo acetoacetyl aminopyridine chelates. Thermochimica Acta 159: 43-54.
- Mustafa I, Ameer B, Naser S, Wasan A, Ali H, et al (2016) Study on Optical Properties of PVC-2,5di (2-Pyrrole hydrazone) 1,3,4-thiadiazole Complexes. Res J Pharm Biol Chem Sci 7(5): 2347-2355.
- Coudert P, Albusson E, Boire JY, Bastide P (1994) Synthesis of pyridazinone derived compounds with promising antiplatelet effects and investigation of their biological activities. Eur J Med Chem 29: 471.
- Sliverstien R, Bassler G, Morrill T (2005) Spectrometric Identification of Organic Compounds. [7th Edn], John-Wiley, New York, USA.
- Adans DM (1967) Metal-Ligand and related Vibration. Edward Arnold, London 33: 310.
- Rahman SMF, Ahmad J, Haq MM (2009) Tris-(salicylaldehyde)- complexes. J Inorg Nucl Chem & J scientific Research 4(4): 229-234.






























