Antioxidant and Antiapoptotic Effects of Fraxetin against Lead Induced Toxicity in Human Neuroblastoma Cells
Challa Suresh1 and Prasanna K Dixit2*
1Department of Biochemistry, National Institute of Nutrition, India
2Department of Zoology, Berhampur University, India
Submission: December 06, 2017; Published: January 03, 2018
*Corresponding author: Prasanna Kumar Dixit, Post Graduate Department of Zoology, Berhampur University, Bhanja Bihar, Odisha, India, Email: drdixit2001@gmail.com
How to cite this article: Challa S, Prasanna K D. Antioxidant and Antiapoptotic Effects of Fraxetin against Lead Induced Toxicity in Human Neuroblastoma Cells. Organic & Medicinal Chem IJ. 2018; 5(1): 555651. DOI: 10.19080/OMCIJ.2018.04.555651
Abstract
Exposure to lead (Pb), a toxic heavy metal produces a wide range of adverse health effects. Both adults and children can suffer from the effects of Pb-poisoning, but childhood Pb-poisoning is much more frequent. Low-level Pb-exposure can result in reduced IQ, learning disabilities, attention deficit disorders, behavioural problems, stunted growth, impaired hearing, and kidney damage. Oxidative stress is proposed as an intracellular mechanism in Pb-induced toxicity, suggesting the fact that antioxidants might play a very important role in the treatment of Pb- poisoning. The present study was designed to investigate whether fraxetin (FXT), an important coumarin has a beneficial effect on human SH- SY5Y neuroblastoma cells exposed to Pb. In the present study, the cells were exposed to different concentrations (0.01-10 mM) of Pb for 48 hours to determine the concentration (IC50 = 5 mM) at which 50% inhibition in the viability of cells.
A significant decrease in the levels of glutathione (GSH), a critical intracellular antioxidant, was observed at all the Pb concentrations. At 5 mM Pb the cells also exhibited a multi-fold increase in Prostaglandin-E2 (289%) and caspase-3 activity (620%) levels. Pre-treatment with 25 mM FXT significantly reversed the effect of on GSH content and caspase-3 activity. The data suggest that some of the neurotoxic effects of Pb may be partly mediated by oxidative stress and apoptosis and pre-treatment with FXT can prevent these effects. The present study asserts the neuroprotective effect of FXT during Pb-induced toxicity in human neuroblastoma cell cultures.
Keywords: Lead; Fraxetin; Neuroblastoma Cells; Glutathione; Caspase-3; Prostaglandin E2
Introduction
Environmental exposure of Lead (Pb) may leads to alterations in the health aspects [1,2] of a normal human being. Different age groups including the old and child age are the frequent sufferers because of the elevated levels of Pb in the blood [3-6]. Brain is the most affected tissue due to the toxicity being generated by Pb [7]. Neuronal toxicity associated with memory, cognitive dysfunction, aggressive behaviour and lack of attention [8] are the typical characteristics of the Pb induced toxicity. School age group children are the most affected group of population because of their growing stage of life associated with developmental progress of nervous system and parts of the brain, which lead to the behavioural changes, learning disabilities, reduced IQ, stunted growth, hearing problems and even pathology associated with kidney dysfunction [9]. Increased exposure of Pb may leads to mental retardation, numbness, changes in the impulse, often ends with death, alterations in the blood pressure, imbalance in the fetal growth, issues associated with pain in the joints and irritation.
Neurological effects include peripheral neuropathy, fatigue / irritability, impaired concentration, hearing loss, wrist / foot drop, seizures and encephalopathy. Morphologically Pb affects axonal and synaptic elaboration; neurochemically it substitutes calcium that results in altered calcium channel and NMA receptor function, while metabolically it causes oxidative stress and damages the developing brain [10-12]. Despite several decades of research on the neurotoxicology of Pb and its continued prominence as a major environmental and occupational health hazard, the mechanisms of its toxic action in the nervous system are still unknown [13] and to date there is no protective drugs/ agents in treating the Pb-poisoning in the clinical practice. Present study examines the neuroprotective effects by Fraxetin (FXT), an important coumarin against the Pb-induced toxicity. Coumarins comprised a group of phenolic compounds widely distributed in natural plants and they have recently attracted much attention because of their pharmacological activities.
They have multiple biological effects including anticarcino- genic, anti-inflammatory, antioxidant, antibacterial, antiviral and antithrombotic effects [14,15]. Many coumarins, such as FXT, showed scavenging activity against reactive oxygen species [16] and inhibit lipid per oxidation in rat brain [17-19]. Although the physiological benefits of coumarins have been largely attributed to their antioxidant properties in plasma, coumarins may also protect cells from various insults. Antioxidant effects of phenolic compounds have been identified, where as there is a lack of evi-dence to support the relationship between FXT and atiapoptotic inducing activities during Pb induced toxicity. Therefore, FXT is tested for the nuero-protective effects if any in the Pb exposed brain cells.
Materials and Methods
a) Materials: RPMI-1640 medium, OPI was obtained from GIBCO Life Technologies. Fetal Bovine Serum, Penicillin and Streptomycin and Phosphate Buffered Saline were procured from CELLGRO, Mediatech. The kits MTT cell proliferation (ATCC, Manassas, VA, USA), Glutathione (Dojindo Molecular Technologies, Gaithersburg, MA,, USA), Prostaglandin E2 (Amersham Biosciences Piscataway, NJ, USA) and Caspase-3 (Biovision, Mountain View, CA, USA) were used to perform the experiments. All other chemicals were of analytical grade were purchased from Sigma.
b) Cell Culture: RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum, 50 μ g/ml penicillin- streptomycin and OPI (150 μ g/ml oxaloacetate, 50 μ g/ml pyruvate and 0.2 U/ml insulin) were used to grow the brain cells in a humidified air/5% CO2 incubator at 370C.
c) Cell viability determination: Viability of the cells were assessed using the trypan blue exclusion method, where the stained cells represents the dead ones were counted as described by Black et al. [20]. The number of stained cells was subtracted from the total count in order to determine the percentage of viable cells.
d) Exposure of cells to Pb and Fraxetin: The 2 x 104 cells per well were treated with different concentrations of Pb acetate (0.01 mM -10mM) and then pre-treated with FXT (0100 mM). Further, cells were exposed to 1C50 concentration of Pb in the presence or absence of FXT for 48 hours and the cell proliferation was determined via MTT reduction assay.
e) MTT assay: Various concentrations of Pb in the presence or absence of FXT was added to the cells and incubated for 48 hours and 10 μl of MTT reagent was added to the culture and incubated in the dark for four hours at 370C. Cell lyses was done by the addition of 100 μl Detergent reagent and kept in the dark for two hours at room temperature. The relative amount of MTT reduction was determined based on the absorbance measured at 570 nm.
f) Measurement of Glutathione content: 5 x 105 cells were centrifuged and the obtained suspension was washed with PBS, then lysed and treated with 10mM HCl and 5% sulphosalycilic acid respectively. The cells were centrifuged at 8000 x g for 10 min and to the supernatant 20 ml of enzyme working solution, 140 ml of coenzyme working solution and 20 ml of either one of the standard solution or the sample solution were added. The plate was incubated at room temperature for 10 min and the optical density was measured at 415 nm and compared with a standard GSH curve.
g) Determination of Caspase-3 activity: Cells were pretreated with Pb in the presence or absence of FXT and resuspended in the 50 l of cell lysis buffer and incubated for 10 min on ice and then centrifuged at 10,000 x g for 1 min. To the supernatant, 50 μl of the reaction buffer containing 10mM dithiothreitol and 5 μl of the 4mM DEVD- pNA substrate was added and incubated at 37oC. After two hours absorbance was measured at 405 nm.
h) Prostaglandin E2 measurement: About 104 to 105 cells/well were seeded and the plate was incubated in a 5% CO2 incubator at 370C. The cells were treated with Pb in the presence or absence of FXT and continued the incubation for 48 hours. Then, 20 μl of buffer A (2.5% Dodecyltrimethylammonium Bromide in 0.1 M Phosphate buffer pH 7.5) and lysed by simple agitation. 50 μl of lysate was transferred to goat anti-mouse IgG coated plate and added 50 μl of lysis reagent. Then, 50 μl of diluted PGE2 antibody and 50 l of diluted conjugate were added to the plate and incubated at room temperature for one hour The cells were washed and added 150 μl of enzyme substrate (3, 3', 5,5' Tetramethylbenzidine) and the reaction was stopped by the addition of 100 μl of μ1 M sulfuric acid after 30 min. Absorbance was measured at 450 nm and compared with appropriate standards.
Data Analysis
Data were expressed as mean ± SD of at least four determinations from each group, repeated at least three times in different occasions. Statistical analysis was performed using one-way analysis of variance (ANOVA) and the statistical significance was assumed at p<0.05.
Results
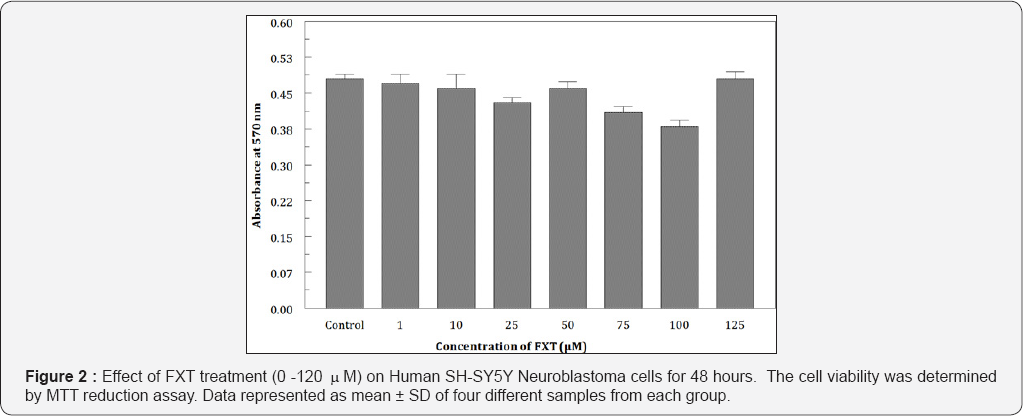
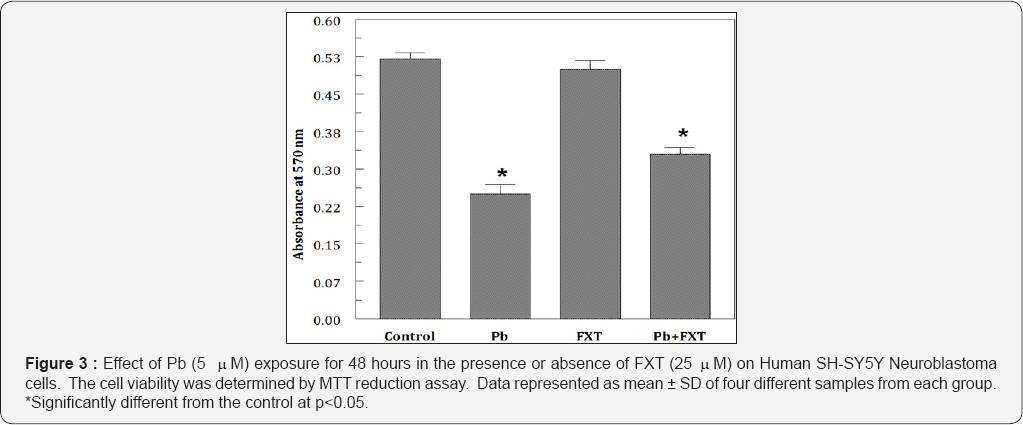
Cells were exposed to different concentrations of Pb (0.01 mM-10 mM) for 48 hours. Pb significantly inhibited the cell proliferation even at low (0.01 mM) concentrations. When treated, Pb reduced the cell viability in a dose dependent manner from 93.4% to 25.5% (Figure 1). IC50 was observed to be at 5 mM Pb. To examine the effects of FXT (0-100 mM) alone, the cells were treated for 48 hours with different concentrations of fraxetin. Results indicate that, FXT has not showed any significant effect on the viability of the cells (Figure 2). Furthermore, protective effects was observed when pretreated with 25 mM of FXT followed by 5 mM Pb for 48 hours (Figure 3) as evidenced from the morphological changes observed in the cells. To observe the effects of Pb on oxidative stress intracellular content of GSH was measured. The intracellular levels of GSH were significantly (p<0.05) altered in the in the Pb exposed group in a dose dependent manner (Figure 4).


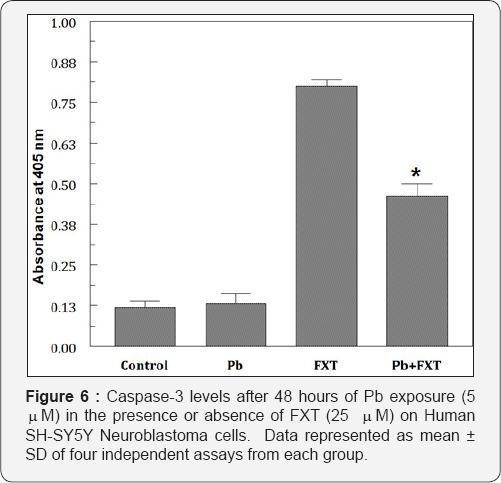
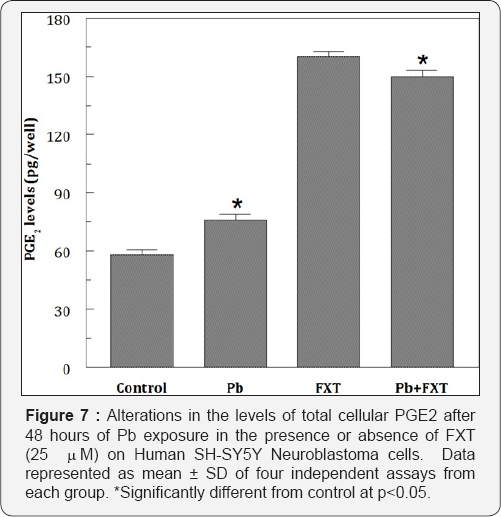
At 10 mM of Pb exposure the maximum reduction in GSH levels were observed and when FXT (5 mM-100mM) alone was exposed for 48 hours there were no alterations in the levels of GSH. But, there was an elevation in the levels of GSH when pretreated with FXT to the Pb exposed cells (Figure 5). Pb but not FXT significantly elevated (289%of the control) the PGE2 levels. Pb but not FXT increased the caspase-3 activity by several fold (Figure 6). The pretreatment with 25 mM FXT followed by Pb (5 mM) exposure resulted in a marginal decrease (253% of the control) in the PGE2 levels (Figure 7). However, FXT pretreatment significantly decreased the Pb-induced increased caspase-3 activity.

Discussion
Recent evidences from our own study reveal the fact that, Pb induces toxicity even at the low levels in the SHSY5Y neuroblastoma cells [21]. It was also established that, oxidative stress and apoptosis are the mechanisms involved in the Pb induced toxicity apart from other mechanisms. As we established in our studies, we observe that Pb even at low levels of 1 ( M was able to reduce the cell viability. However, in the presence of the FXT the viability of the cells were preserved inspite of the Pb exposure which indicated the fact that the cells were protected from undergoing death. This particular event was very much visible when the cells were viewed under the microscope. Also, the study has reveled the fact that, FXT pretreatment markedly attenuated the well defined morphological changes which were not the case in the Pb exposed cells. In addition to having a role in intra and extra cellular signaling, these reactive molecular species may initiate damaging biochemical reactions [22,23].
In response to such damages, a complex antioxidant defence has developed, and natural antioxidants comprise an important role in the defence. Hence forth it is the need of the hour to find alternative and prospective antioxidants which are easily available in the natural food material and preparations from the different endangered plant extracts for therapeutic applications. Since Pb is causing oxidative stress [21], these types of compounds may play an effective role as antioxidants in nullifying the developed toxicity [24]. Further, Glutathione redox cycle is a major endogenous protective system and an important component of the antioxidant machinery particularly the nervous system [25]. Earlier reports indicate that depletion of cellular GSH leads to the accumulation of ROS and loss of mitochondrial functions [26]. Fraxetin, has been shown to be capable of elevating intracellular GSH in a Drosophila melanogaster experimental model [2 7] and in liver supernatant from male mice [28,29].
However, inducibility of GSH by FXT in the Pb exposed cells in neuronal cells has not been reported in the literature. In the present study exposure of the cells to Pb caused the reduction in the intracellular levels of GSH. But, when the Pb was premixed with FXT, the levels of GSH has been drastically restored, indicating the fact that FXT is playing an important role as an antioxidant in protecting the cells from the oxidative stress. Apoptosis also known as programmed cell death, is a crucial biological process involved in maintaining development and tissue homeostasis. Inappropriate stimulation or inhibition of stimulation or inhibition of apoptosis can lead to several physiological imbalances and disorders. In the present study, FXT treatment significantly reduced the Pb-induced toxicity on the cells, where the cell viability and proliferation of the cells were restored. The results indicate the fact that caspase-3 levels has increased in multifold levels upon the Pb exposure, may be a resultant of toxic induction by Pb. Hence, the increase in cell death during Pb exposure could be attributed to increased caspase-3 activity and /or induced oxidative stress as evidenced from significant reduction in GSH levels. Whereas, FXT, a known antioxidant can combat these toxic effects effectively and playing an important role in protecting the cells. The results confirm the earlier reports on FXT as an effective antiapoptototic and as neuroprotective agent [30-33].
Triggering pathways associated with cycloxygenase and the ultimate synthesis of PGE2 is an important event that leads to the damage to the neurons [34]. At low concentrations Pb induced the release of significant levels of PGE2 probably through cycloxygenase pathway. Further, pre-treatment of the SH-SY5Y cells with FXT has not elicit any significant changes in PGE2 levels. From the results of this study, it is clear that the different mechanisms like Pb induced oxidative stress with the decreasing GSH levels, inducing the caspase-3 activity and probably the activation of cycloxygenase by increasing the levels of PGE2. The results suggests that FXT can reverse the activated mechanisms and especially GSH related antioxidant effects of FXT could be regarded as a beneficial property of this as a neuroprotective drug. In conclusion, fraxetin can reduce the Pb induced neuronal loss involving GSH, Caspase-3 and PGE2 mechanisms.
Conclusion
Pb exposure has resulted in the development of oxidative stress, apoptosis and inflammation. These effects could be deleterious to the brain cells in developing neurotoxicity. Fraxetin exposure has protected the cells from the damage and can effective against the lead induced neurotoxicity.
Acknowledgements
This research work was supported by ICMR Grant NO.58/57/2012-BMS.
References
- Regan CM (1989) Pb impaired development. Mechanism and threshold values in the rodent. Neurotoxicol Neuroteratol 533-537.
- Silbergeld E K (1991) Mechanisms of Pb neurotoxicity or looking beyond the lamp post. FASEB J 6: 3201-3206.
- Bellinger D and Dietrich K N (1994) Low lead explosure and cognitive function in children. Paediatric Ann 23: 600-605.
- Chetty CS, Reddy GR, Murthy KS, Thomson J, Sajwank, et al. (2001) lead exposure alters the expression of neuronal nitric oxide syntheses in rat brain. Int J Toxiol 20: 113-120.
- Wilson MA, Johnston MV, Goldstein GW, Blue, ME (2000) Neonatal lead exposure impairs development of rodent barrel field cortex. Proceedings of the National Academy of Sciences 97(10):5540-5545.
- Basha MR, Wei W, Brydie M, Razniafshari M, Zamia NH, et al. (2003) Lead-induced developmental perturbations in hippocampal Sp1-DNA- Binding are prevented by zinc supplementation: in vivo evidence for Pb and Zn competition. Intl J Dev Neurosic 21:1-2.
- Rafaliwska U, Struzynska L, Dabrowska Bouta B, Lenkiewicz A (1996) Is lead toxicosis a reflection of altered energy metabolism in brain synaptosomes? Acta neurobiol. Exp (Warsz) 56: 611-617.
- Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environmental Toxicology and Pharmacology 48: 203-213.
- Bellinger DC, Stiles KM (1993) Epidemiologic approaches to assessing the developmental toxicity of Pb. Neurotoxicology 14: 151-160.
- Gurer H, Ozyguner H, Saygin E, Eral N (2001) Antioxidant effect of taurine against lead induced oxidative stress. Arch Environ Contam Toxicol 41(4): 397-402.
- Scortegagna M Chikale, E Hamaker I (1998) Lead exposure increases oxidative stress in serum deprived C14 mesencephalic cultures. Role of metallothionine and glutathione Retor neurol necurosc 12 (2-3): 95101.
- Toscano CD, Guilarte TR (2005) Lead neurotoxicity from exposure to molecular effects. brain Res Rev 49 (3): 529-554.
- Bressler jp, Goldsteion GW (1991) Mechanisms of lead neurotoxicity. Biochem Pharmacol 41: 479-484.?
- Liu ZQ Yu W, Liu ZL (1999) Antioxidative and peroxidative effects of coumain derivatives on free radical initiated and photosensitized peroxidative of human low density lipoprotein. Chem Phys Lipids 103(1-2): 125-135.
- Fylakatakidou KC, Hadjipavlou Litina DJ, Litinas K E, Nicdailes DN (2004). Natural and synthetic coumarin derivatives with antiinflammatory/ antioxidant activities. Curr Pharm Res 10(30): 38133833.
- Paya, M Hallimell, B Haoult JR (1992) Peroxyl radical scavenging by a series of coumarins. Free Radic Res Commn 17(5): 293-298.
- Maritin Aragon S, Benedi JM, Villar AM (1997) Modifictions on antioxidant capacity and lipid peroxidation in mice under fraxetin treatment. J Pharma Pharmacol 49(1): 49-52.
- Paya M, Ferrandiz ML, Miralles F, Montesinos C, Ubeda A, et al. (1993) Effects of coumarin derivatives on superoxide anion generation. Arzne imitte forschung 43(6): 655-658.
- Paya M, Goodwin PA, Heras B, Hoult J R (1994) Superoxide scavenging activity in leucocytes and absence of cellular toxicity of a series of coumarins. Biochem Pharmacol 48(3): 445-451.
- Black L, Berenbaum MC (1964) Factors affecting the dye exclusion test for cell viability. Exp Cell Res 35: 9-13.
- Chellu S Chetty, Mohan C Vemuri, Khamisi Campbell, Challa Suresh (2005) Lead induced cell death of Human Neuroblastoma Cells involves GSH deprivation. Cell and Molecular Biology Letters 10: 413-423.
- Halliwell B (1999) Antioxidant defense mechanisms: from the beginning to the end (of the beginning ). Free Radic Res 31: 261-72.
- Briviba K, Klotz LO, Siera H (1997) Toxic and signaling effects of phytochemically or chemically generated singlet oxygen in biological systems Boil Chem 378: 1259-65.
- Soto Otero, R Mendez Alvarez E, Hermida Ameijeiras A, Munoz Patino AM, Labandeira Garcia JL, et al. (2000) Antioxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants. Potential implications in relation to the pathogenic of Parkinson's disease. J Neurochem 74(4): 1605-1612.
- Seyfried J, Soldner F, Kunz W S, Schulz J B, Klochgether T, et al. (2000) Effect of 1-methyl-4-phenyl pyridinium on glutathione in rat pheochromocytoma PC 12 cells. Neurochem Int 36(6): 489-497.
- Bharat S, Cochran BC, Hsu M, Lin J Ames B N, et al. (2002) Pretreatment with R-lipoic acid alleviates the effects of GSH depletion in PC12 cells. Implications for Parkinson's disease therapy. Neurotoxicology 23: 479486.
- Fernandez Puntero B, Barroso 1, 1glesias 1 Benodi J, Villar A (2001) Antioxidant activity of fraxetin.; in vivo and ex vivo parameters in normal situation verses induced stress. Boil Pharm Bull 24: 777-784.
- Martin Aragon S, Benedi J M, Villar A M (1997) Modification on antioxidant capacity and lipid peroxidation in mice under traxetin treatment. J Pharm Pharmacol 49: 49-52.
- Martin Argon S, Benedi JM, Villar AM (1997) Effects of fraxetin on glutathione redox status. Z Natur forsch 52(2): 55-59.
- Hou A, Cymbalyuk E, Hsu SC, Xu M, Hsu YT, et al. (2003) Apoptosis modulatory activities of transiently expressed Bcl-2: roles in cytochrome C release and Bax regulation. Apoptosis 8(6): 617-629.
- Molina Jimenez MF, Sanchez Rens, MI Benedi J (2003) Effect of fraxetin and myricetin on rotenone induced cytotoxicity in SH-SY5Y cells Comparision with N-acetyl cysteine. Eur J Pharmacol 472: 81-87.
- Molinc Jimenz MF, Sanchez Reus MJ, Andres D, Cas Cales M, Benedi J, et al. (2004) Neuroprotective effect of fraxetin and myricitin against rotenone -induced apoptosis in neuroblastoma cells. Brain Res 1009: 9-16.
- Martin Aragon S, Benedi J, Villar A (1997) Effect of fraxetin on glutathione redox status. Naturforsch 52: 55-59.
- Bate C, Rutherford S, Gravenol, M, Reid S, William A, et al. (2002) Cycloxygenase inhibition protect against prion induced neurotoxicity in vivo. Neuroreport 13(15): 1933-1938.






























