Abiotic Fluorescence Sensor for Aluminium ions
Kishor Kumar Suryavanshi and Subrata Jana*
Department of Chemistry, Indira Gandhi National Tribal University (Central University), India
Submission: December 20, 2017; Published: January 03, 2018
*Corresponding author: Subrata Jana, Department of Chemistry, Indira Gandhi National Tribal University (Central University), Amarkantak, MP, India, Tel: (+91) 7869494850; Email: jana.s.oc@gmail.com
How to cite this article: Kishor K S, Subrata J. Abiotic Fluorescence Sensor for Aluminium Ions. Organic & Medicinal Chem IJ. 2018; 4(5): 555650.DOI10.19080/OMCIJ.2018.05.555650
Abstract
In the present mini review, couple of recent literature for the design and synthesis of artificial chemosensor for Al3+ is being incorporated. These are very efficient sensor in terms of strong binding affinity along with high detection limit.
Introduction
Aluminium is the third most abundant (8.3% by weight) metallic element in the earth. Various compounds containing Aluminium are immensely used in the different type of industry like, paper, in dye production, in the textile industry [1-3]. Aluminium salts are also used as a component for the preparation of many cosmetics and in alimentary industry [4,5]. Many drug molecules are also prepared using aluminium compounds for human and veterinary medication [6]. Among them, buffered aspirin containing aluminium glycinate is commonly used as an analgesic [7]. Accumulation of excessive amounts of this metal damages the kidney, central nervous system causing Parkinson's disease [8,9]. It reduces total bone and matrix causing osteoporosis, osteomalacia [10,11]. It also retarded plant growth and damage water body ecosystem by killing fish due to enhance acidity of the waters [12-14]. The maximum recommended limit by the FAO/WHO Joint Expert Committee on Food Additives for daily intake of aluminium is 3-10 mg per day per body mass. So aluminium ions have a very adverse effect on living organism when it crosses the permissible limit. Beside it is highly responsible for polluting soil, water and even ground water. The aluminum containing compounds present on earth surface are very much susceptible to any type of acidic contamination which may come from industrial effluent or rain water.
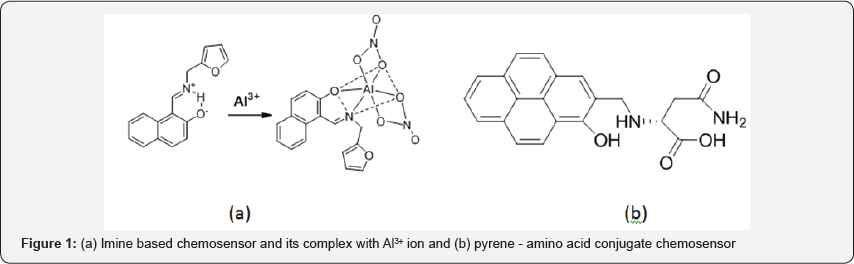
Therefore, development of convenient and selective methods for determination of Aluminium is highly desirable [10]. Several methods have been reported in the literature for the estimation and detection of Al3+ but the spectrofluorimetry procedure has received considerable attention in recent years due to its easily detectable signals upon recognition of metal ions with high sensitivity and selectivity [15-19]. Though there are numerous reports have been published but only couple of interesting examples are mentioned herewith. Sen et al. [20] has reported an imine based water soluble chemosensor and its application in living cell imaging ( Figure 1). This probe performed sensing of Al3+ in physiological pH. Another probe based on pyrene-amno acid conjugate has been reported by Sun et al. [21]. This sensor is effective to detect both Al3+ and H+. Beside there is some strong solvent effect during sensing process.
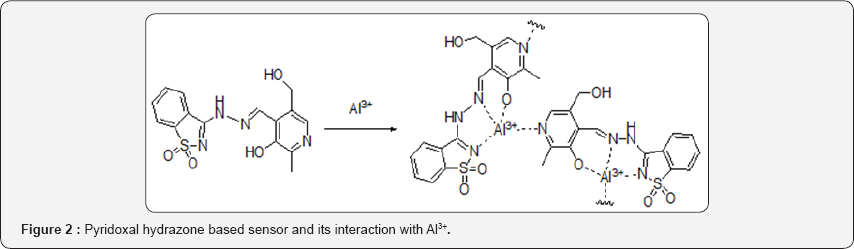
The chelation enhanced fluorescence effect has been observed during the sensing of Al3+ by a tailor made pyridoxal hydrazone based receptor (Figure 2). This chemosensor shows very high association constant as well as high detection limit towards Al3+ [22]. Along with different chemosensor with wide variety of fluorophore rodamin B is an excellent tool for the designing of a different class of sensor with very high binding capabilities and detection limit [23]. Beside Maity et al. reported a pyrrolidine constrained bipyridyl-dansyl fluoroionophore for the sensing of Al3+ ion with very high binding affinity [24].
Conclusion
Aluminium ion has prominent role in different biochemical pathways and environmental issues. So design and synthesis of efficient chemosensor for Al3+ is a very important area of research and draw much attention from the scientific community. Though it is always very challenging for the researcher to design and synthesize a sensor which can work in either physiological or aqueous environment. Here couple of different sensor is being included though there is numerous reports for this purpose are omitted due to space.
References
- Miller WS, Zhuang L, Bottema J, Wittebrood AJ, Smet PDe, et al. (2000) Recent development in aluminium alloys for the automotive industry. Mater Sci Eng A 280: 37-49.
- Doherty RE (2000) Environ Forensics 1: 83-93.
- Ciardelli G, Ranieri N (2001) The treatment and reuse of wastewater in the textile industry by means of ozonation and electroflocculation. Water Res 35(2): 567-572.
- Humphreys S, Bolger PM, Zatta PF, Alfrey AC (1997) World Scientific Singapore 226-237.
- Yokel RA (2008) Aluminum Bioavailability from basic sodium aluminum phosphate, an approved food additive emulsifying agent, incorporated in cheese. Food Chem Toxicol 46(6): 2261-2266.
- Greger JL (1997) Aluminum exposure and metabolism. Crit Rev Clin Lab Sci 34:439-474.
- Lione A (1985) Gen. Pharmacol 16:223-245.
- Sorenson JRJ, Campbell IR, Tepper LB, Lingg RD (1974) Aluminum in the Environment and Human Health. Environ Health Perspect 8: 3-95.
- Flaten TP (2001) Aluminium as a risk factor in Alzheimer's disease, with emphasis on drinking water. Brain Res Bull 55(2): 187-196.
- Baral M, Sahoo SK, Kanungo BK (2008) Tripodal amine catechol ligands: a fascinating class of chelators for aluminium(III). J Inorg Biochem 102(8): 1581-1588.
- Azadbakht AR, Rashidi S (2014) Spectrochim Acta A 127:329-334.
- Gupta VK, Jain AK, Maheshwari G (2007) Aluminum (III) selective potentiometric sensor based on morin in poly(vinyl chloride) matrix. Talanta 72(4): 1469-1473.
- (a) Alvarez E, Fernandez ML, Monterroso C, Fernandez SM (2005) Forest Ecol Manage 211:227-239. (b) Barcelo J, Poschenrieder C (2002) Environ Exp Bot 48:75-92.
- Alstad NEW, Kjelsberg BM, Vollestad LA, Lydersen E, Poleo ABS (2005) Environ Pollut 133:333-342.
- Saleh MB, Hassan SSM, Gaber AAA, Kream NAA (2001) Anal Chim Acta 434:247-253.
- Downard AJ, Sullivan BO, Powell KJ (1997) Anal Chim Acta 345:5-15.
- Lian H, Kang Y, Bi S, Arkin Y, Shao D, Li D, Chen Y, Dai L, Gan N, Tian L (2004) Talanta 62:43-50.
- De Silva, AP, Fox DB, Huxley JM, Moody TS (2000) Coord Chem Rev 205:41-57.
- Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205: 3-40.
- Sen S, Mukherjee T, Chattopadhyay B, Moirangthem A, Basu A, Marek J, Chattopadhyay P (2012) A water soluble Al3+ selective colorimetric and fluorescent turn-on chemosensor and its application in living cell imaging. Analyst 137(17): 3975-3981.
- Sun Y, Wang Y-W, Peng Y (2012) Org Lett 14:3420-3423.
- Keji'k Z, Kaplanek R, Havli'k M, Bri'za T, Vavrinova D, Dolensky B, Martasek P, Kral V (2016) J of Luminescence 180: 269-277.
- Liu T, Wan X, Yao Y (2018) Sensors and Actuators B: Chemical 254: 1094-100.
- Maity D, Govindaraju T (2010) Pyrrolidine constrained bipyridyl- dansyl click fluoroionophore as selective Al3+sensor. Chem Commun 46(25): 4499-4501.






























