Synthesis, Characterization & Biological Evaluation of Mutual Prodrugs of Some Selected NSAIDs conjugated with different antioxidant via different amino acids
Sharma N*
K N Modi Institute of Pharmaceutical Educational & Research, India
Submission: October 31, 2017; Published: November 27, 2017
*Corresponding author: Sharma N, K N Modi Institute of Pharmaceutical Educational & Research, India Email: nidhi.shar27@gmail.com
How to cite this article: Sharma N. Synthesis, Characterization & Biological Evaluation of Mutual Prodrugs of Some Selected NSAIDs conjugated with different antioxidant via different amino acids. Organic & Medicinal Chem IJ. 2017; 4(4): 555642.DOI:10.19080/OMCIJ.2017.05.555642
Abstract
Some selected non-steroidal anti-inflammatory drugs (Aceclofenac, Diclofenac), have been conjugated with antioxidant (Guaiacol, Menthol) via Amino acid spacer (Phenylalanine, Glycine) which has anti-inflammatory, cytoprotective and immunomodulatory properties; therefore this conjugation will synergize the effect of the parent NSAIDs as anti-inflammatory drugs and reducing their gastric side effects. Four compounds have been synthesized andthe structure was confirmed by using IR, NMR (1H-NMR) & Characterized by some physicochemical properties including Melting point. IR data were recorded on Shimadzu FT-IR Spectrophotometer using KBr pellets method & 1H-NMR spectra in DMSO on Bruckerspectrospin-300 MHz using TMS as internal standard. The purity of the synthesized compounds was checked by its TLC. Developing solvents used for the process was [Methanol: Chloroform (2.5ml: 7.5ml)]. TLC plates were made by using Silica gel G & Silica gel GF.
Results & Discussion

b. Antimicrobial Activity: As shown in Table 2.

Conclusion: A mutual prodrug is a form of drug where two pharmacologically active agents are attached to each other in such a way that they acts as a promoiety/carrier for each other. In this study, titled compounds act as a mutual prodrug, all compounds showed the significantly antiinflammatory effect, antimicrobial activity and antioxidant activity. Further, the study would be concluded the mechanism of action and in-vivo study to predict the efficacy of these compounds on rats towards anti-inflammatory effect.
Keywords: NSAIDs; Mutual prodrug; Ulcerogenicity; Antioxidants; Amino acids
Abbreviations: NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; COX: Cyclooxygenase; GI: Gastro Intestinal; DMF: Di Methyl Formamide; DMAP: Di Methyl Amino Pyridine
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most widely used medicines in the world, because of their analgesic, anti-inflammatory and antipyretic activities along with this it is alsomostcommonlyusedmedications forthe treatmentofthesigns and symptoms of arthritis through inhibition of prostaglandin biosynthesis [1,2]. Prostaglandins are lipid compounds formed when cyclooxygenase (COX) enzymes metabolize Arachidonic acid released from membrane Phospholipids [3]. Prostaglandins mediate a range of normal biological functions including gastric protection, renal homeostasis, vascular homeostasis, uterine function, embryo implantation and labour, regulation of the sleep wake cycle, body temperature and inflammation [4,5].
There are several drawbacks with NSAIDs, in which the most common side effect of NSAIDs is a gastrointestinal (GI) complications; it is a well-accepted fact that the GI side effect of acidic NSAIDs is a result of two different mechanisms; direct and indirect irritation of the gastrointestinal (GI) tract [6,7]. NSAIDs cause a dual assault on the GI tract: the acidic molecules directly irritate the gastric mucosa, and inhibition of COX-I that reduce the levels of protective prostaglandins [8].Inhibition of prostaglandin synthesis in the GI tract causes increased gastric acid secretion, bicarbonate secretion, mucus secretion and diminished trophic effects on epithelial mucosa [9].
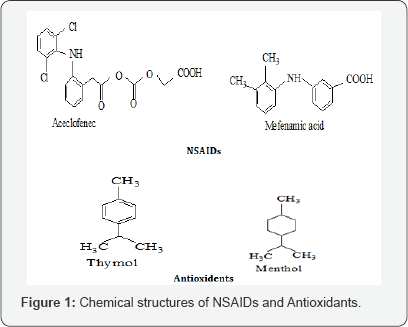
Considerable attention has been focused on the development of bioreversible derivatives (prodrugs) to temporarily mask the acidic group of NSAIDs as a promising means of reducing or abolishing the GI toxicity [10]. Prodrugs are pharmacologically inactive derivatives of active agents, which undergo chemical and/ or enzymatic biotransformation resulting in the release of active drug after administration. The metabolic product (i.e. parent drug) subsequently elicits the desired Pharmacological response [11,12]. According to prodrug concept i have been synthesized prodrug of some NSAIDs; naproxen and mefenamicacid; which are represented in figure 1 bycoupling with antioxidant [thymol or menthol] by using alanine amino acid as spacer.
The use of antioxidants will be over advantage; it will be equivalence free radicals or reactive oxygen species (ROS) that plays significant role in the formation of gastric ulceration associated with NSAID therapy [13], according to this observation antioxidants may be used to prevent NSAIDs induced gastric ulcers [14]. Using of alanine amino acid as spacer exhibits number of advantages it had broad spectrum activity as; antiinflammatory, cytoprotective and immunomodulatory properties and therefore would synergize the effect of non-steroid antiinflammatory prodrugs for enhancing its anti-inflammatory potential and reducing the gastric side effect [15,16]. Prodrugs of agents that contain carboxylic acid or alcohol functionalities can often be prepared by conversion in to an ester. This is the most common type of prodrug because of the ease with which the ester can be hydrolyzed to give the active drug [17]. Therefore; antioxidant was esterified with carboxylic group of alanine.
Derivatization of agents that contain carboxylic group with amines to give amides has not been widely used as a prodrug strategy because of the high chemical stability of the amide linkage and lack of amidase enzymes necessary for hydrolysis [18]. Therefore, amidation of NSAIDs with alanine was hoped to make them more stable in the stomach as amidases that bring upon the hydrolysis of amide bond are present in the intestine [19,20]. However, prodrugs hydrolysis aren't prerequisite in the designed analogues as it was shown that ester and amide derivatives of NSAIDs are potent cyclooxygenase-2 inhibitors and retain the anti-inflammatory activity of the parent NSAIDs [21,22] (figure 2).
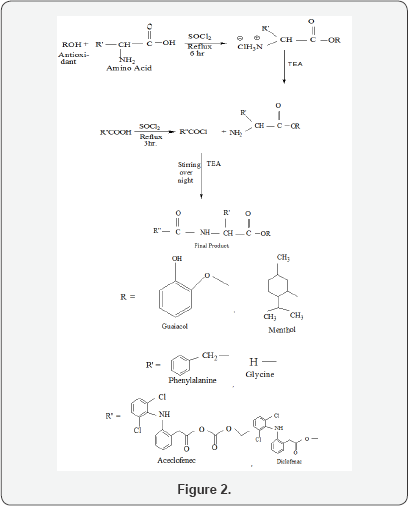
Compound I: if R= Guaiacol, R'= Glycine, R"= Aceclofenec
Compound II: if R= Menthol, R'= Glycine, R"= Diclofenec
Compound III: if R= Menthol, R'= Phenylalanine, R"= Diclofenec
Compound IV: if R= Guaiacol, R'= Phenylalanine, R"= Aceclofenec
Experimental Procedure
A. Synthesis of Amino acid (Phenylalanine, glycine)- antioxidant (Menthol, Guaiacol) ester hydrochloride compounds
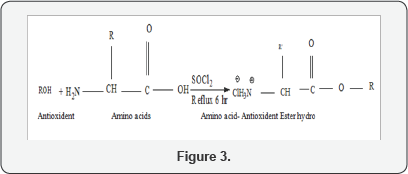
Antioxidant (3.125gm/mol Menthol, 2.483gm/molGuaiacol) (2 mmol) was dissolved in dry acetone (20 ml) with vigorous stirring until all the crystal completely dissolved, Amino acid (phenyl alanine, Glycine) (2 mmol) was added to it with continuous stirring. Then themixture was cooled in ice bath at temperature -5oc. Thionyl chloride (5mmol, 0.4 ml) was added drop by drop to the mixture (the mixture should be maintained with constant stirring at -5oc for 1 hr). After that left with stirring at room temperature for 15 min. Then the mixture was refluxed for 6 h with continuous stirring and the reaction was monitored by evolution of HCl gas which was detected by changing the colour of pH graduated litmus paper into reddish. Solid product was collected after filteration. It was re-crystallized from hot methanol by slow addition of 15-20 ml ether followed by cooling at 0oc. The crystals were collected on the next day (Figure 3).
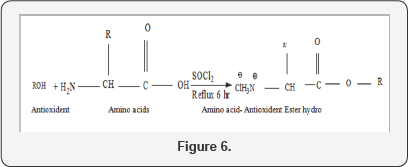
B. Synthesis of Amino acids- antioxidant ester
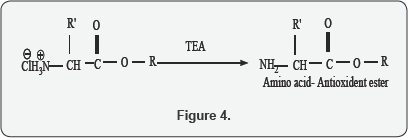
To a suspension of the Alanine-antioxidant ester hydrochloride (5 mmol) in dry CHCl3 (20mL), tri ethyl amine (1 mL, 10 mmol) was added drop wise over a period of 10 min at 0oc with continuous stirring for 2 hr. The reaction mixture was then filtered and the chloroform layer was distilled off to get clear solution. The clear solution was directly used for the next coupling step (Figure 4).
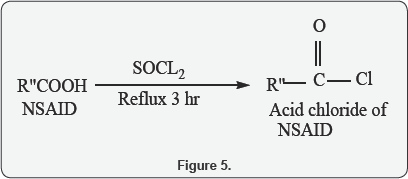
C. General procedure for the preparation of the Acid chloride of NSAIDs
NSIADs (Aceclofenac, Diclofenac) (5 mmol) was dissolved in dry chloroform (20 mL) in a 100 mL round-bottomed flask. Thionyl chloride (15 mmol, 1.1 mL) was added drop wise over a period of 15 min. with cooling on ice bath. The mixture was refluxed for 3hr at 65°C with continuous stirring and monitored by evolution of HCl gas (which is detected by changing the colour of Litmus paper into reddish when placed on the top of condenser) and changing the colour of the solution from colourless into deep yellow. The reaction is often promoted by the addition of a drop of dimethyl formamide (DMF). The excess of thionyl chloride and solvent was removed under reduced pressure and the residue was re-dissolving in dry chloroform (20 mL) and was re-evaporated to give oily residue compound. This compound was directly used for the next coupling step with free Amino acid-antioxidant ester (Figure 5).
D. Synthesis of final compounds
A solution of Amino acid- antioxidant ester was mixed with dry chloroform (15 mL) and, then tri ethylamine (5 mmol, 0.5 mL) was added drop wise with stirring for 20 min. on ice bath. Freshly prepared acid chloride of either NSAIDs (Aceclofenac, Diclofenac) was slowly poured for 50 min. with continuous stirring on an ice bath. Then stir the mixture at room temperature overnight. The reaction can be accelerated by adding a catalytic amount (2-3 drops) of pyridine, or N, N-di methyl amino pyridine (DMAP). Solvents were removed under reduced pressure by using rotary evaporator. The resulting solid product was re- dissolved in ethyl acetate (10 mL) and washed with 5 % aqueous solution of sodium bicarbonate (20 mL), 5% HCl (20 mL) and distilled water (20 mL) and then dried over anhydrous magnesium sulphate, filtered and the solvent was evaporated under reduced pressure to get the final products [23] (Table 3) (Figure 6).
i. IR data of the final compound: The structural features of the synthesized compounds were confirmed by I.R spectral analysis. KBr pellets method was used for the analysis.
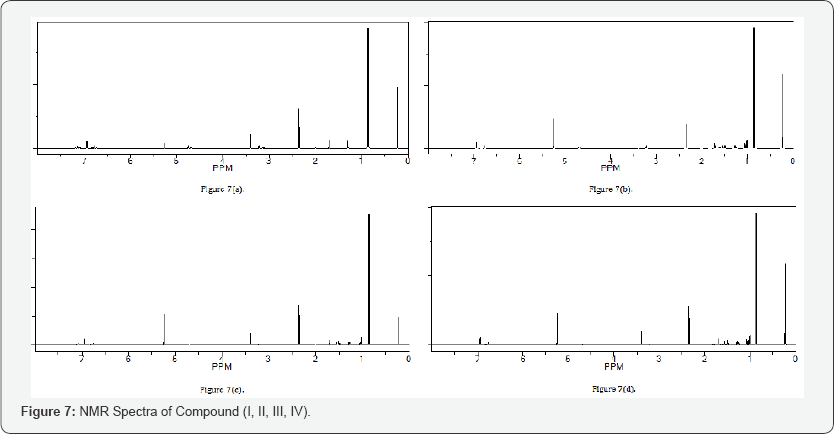
ii. NMR Data: The NMR spectra indicates that N5 compound got singlet at 8 values 7.735 (s, 1H, NH), 6.90 (s, 2H, CSNH2), 7.344-7.6(m, 4H, Aromatic), 2.4 (s, C-NH) (Figure 7).
References
- Laine L (2004) Proton pumps inhibitor co-therapy with nonsteroidal anti-inflammatory drugs-nice or necessary?. Rev Gastroenterol Disord 4 (suppl4): S33-S41.
- Furst DE, Ulrich RW, Prakash S (2012) Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Masters SB, Trevor AJ (Eds.) Basic and Clinical Pharmacology (12th edn.) McGraw-Hill, New York, USA.
- Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31(5): 986-1000.
- Laurence LB, John SL, Keith LP (2008) Goodman and Gilman's the Pharmacological Basic of Therapeutics (11th edn.). The McGraw-Hill Inc, USA.
- HN Jabbour, KJ Sales (2004) Prostaglandin receptor signaling and function in human endometrial pathology. TRENDS in Endocrinology and Metabolism 15(8): 398-404.
- Lenard M Lichtenberger, Yong Zhou, Elizabeth J Dial, Robert M Raphael (2006) NSAID injury to the gastrointestinal tract: evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. J Pharm Pharmacol 58(11): 1421-1428.
- Wallace JL, Cirino G (1994) The Development of GastrointestinalSparing Nonsteroidal Anti-Inflammatory Drugs. Trends Pharmacol Sci 15(11): 405-406.
- Ajaikumar K, Asheef M, Babu B, Padikkala J (2005) The inhibition of gastric mucosal injury by Punicagranatum L (pomegranate). Methanolicextract Journal of Ethnopharmacology 96(1-2): 171-176.
- Simone Rossi (2006) Australian medicines handbook.
- Parmeshwari K Halen, Prashant R Murumkar, Rajani Giridhar, Mange Ram Yadav (2009) Prodrug Designing of NSAIDs. Mini-Reviews in Medicinal Chemistry 9(1): 124-139.
- Kumud M, Manishika S, Seema T (2011) Quest for alternative to NSAIDs Gastropathy: Mutual Prodrugs. International J of Research in Pharmaceutical and Biomedical Sciences 2(4): 1394-140.
- Jolanta B Zawilska, Jakub Wojcieszak, Agnieszka B Olejniczak (2013) Prodrugs: A challenge for the drug development. Pharmacological Reports 65(1): 1-14.
- Chen HY, Lin YC, Hsieh CL (2007) Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem 104(4):1418-1424.
- Chen HY, Lin YC, Hsieh CL (2007) Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem 104(4):1418-1424.
- Anil Samleti, Rajesh Kane, Shrinivas Bumrela, Snehal Dhobale, Atul Thite (2012) Synthesis and Evaluation of Anti-inflammatory activity of Mutual Prodrugs of Aspirin with Amino Acids. International Journal Of Pharmaceutical And Chemical Sciences 1(4): 1343-1349.
- Nelson David L, Cox Michael M (2005) Principles of Biochemistry (4th edn.). WH Freeman, New York pp. 684-685.
- Wilson, Gisvold chapter prodrug.
- Mohan R, Ramaa CS (2007) Ind J Chem 46-B: 1164-1168.
- Lohade AA, Jain KP, Iyer KR (2009) Ind J Pharm Edu Res 43: 140-149.
- Kalgutkar AS, Crews BC, Rowlinson SW, Marnett AB, Kozak KR, et 22. Kalgutkar AS, Rowlinson SW, Crews BC, Marnett L (2002) Amide al. (2000) Biochemically based design of cyclooxygenase-2 (COX-2) derivatives of meclofenamic acid as selective cyclooxygenase-2 USA 97(2): 925-930.
- Kalgutkar AS, Marnett AB, Crews BC, Remmel RP, Marnett LJ (2000) Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J Med
- Kalgutkar AS, Rowlinson SW, Crews BC, Marnett L (2002) Amide derivatives of meclofenamic acid as selective cyclooxygenase-2 inhibitors. J Bioorg Med Med Chem Lett 12(4): 521-524.






























