Antibacterial Activity of a Triterpene Isolated from Combretum Erythrophyllum Ethyl Acetate Fraction
F Mtunzi1*, I Ledwaba1, M Klink2, E Dikio1, P Ejidike1 and V Pakade1
1Department of Chemistry, Vaal University of Technology, South Africa
2Department of Biotechnology, Vaal University of Technology, South Africa
Submission: October 31, 2017; Published: November 21, 2017
*Corresponding author: Fanyana Mtunzi, Department of Chemistry, Vaal University of Technology, South Africa, Email: fanyana@vut.ac.za
How to cite this article: F Mtunzi, I Ledwaba, M Klink, E Dikio, P Ejidike , et al. Antibacterial Activity of a Triterpene Isolated from Combretum Erythrophyllum Ethyl Acetate Fraction. Organic & Medicinal Chem IJ. 2017; 4(3): 555640.DOI:10.19080/OMCIJ.2017.05.555640
Abstract
Combretum erythrophyllum is a member of Combretaceae family, widely used for treatment of venereal diseases. Roots are used as a purgative while dried and powdered gum can be applied to sores. Antibacterial activity ofthe leaves was investigated against Staphylococcus aureus, Escherichia coli, Enterococcus faecalis and Pseudomonas aeruginosa. Activity guided fractionation of the extracts resulted in the isolation of triterpenoid friedelin from acetone crude extract. The structure of the isolated compound was elucidated with the aid of spectral comparison of IR, one and two dimensional NMR experimental values and MS. To the best of our knowledge, this is the first time friedelin was isolated from Combretum erythrophyllum but it has been reported for members of the Combretaceae family like Combretum duarteanum.
Materials and Methods
a. Plant Material
i. Plant collection and treatment: Leaf materials of Combretum erythrophyllum were collected at the University of Pretoria botanical garden (Onderstepoort campus) in January 2010. The plant was identified and authenticated by Prof J N Eloff from University of Pretoria. Leaves were dried in the shade at room temperature for two weeks. Stems and thick veins were sorted and the dried leaves were ground to a fine powder form using a pulveriser and stored in a dry, air tight container away from moisture and direct sunlight.

b. Extraction: The crude extract of Combretum erythrophyllum was suspended in water in a 2 L separating funnel and partitioned using 300 ml of each of the following solvents: Hexane, dichloromethane, water and ethyl acetate in increasing order of polarity [1,2]. Ethyl acetate fraction was found to have more active compounds against tested pathogens (Figure 1).
c. Fractionation and Isolation: Ethyl acetate soluble fraction (10 g) mixed with silica gel was purified by chromatography on silica gel 60 F254 column ($2.0 X 40 cm) eluted successively with gradient mixture of hexane: ethyl acetate [100:0, 98:2, 95:5, 90:10, 85:15, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 0:100]. The eluents were analyzed by TLC (Silica gel 60 F254) (, and similar fractions were combined after TLC analysis. The ethyl acetate extract subjected to successive elution with gradient mixtures gave ten fractions (EA 1-9), (EA 10-16), (EA 17-20), (EA 21-30), (EA 31-48), (EA 49-77) (EA 78-96), (EA 36-43), (EA 97-110), (EA 111128). These fractions were combined based on their TLC profile. Further purification of the fraction (EA 49-77) was performed using glass prep plates coated with silica gel 60 F254 with 95:5 hexane/ethyl acetate mixture as eluent. The melting point (m.p.) was measured using a Microquimica MQAPF-301 Model. The IR spectra were recorded using KBr discs on Perkin Elmer FTIR spectrophotometer model 1275X. The 1H- and 13C-NMR spectra were obtained from Agilent spectrometer in CDCl3 operating at 400 MHz and 125 MHz, respectively. Chemical shifts are shown in 5 values (ppm) with tetramethylsilane as an internal standard. The isolated compound were needle like in appearance (30 mg, Mp 261-263 °C; [α]D25 +2.0°) upon recrystallization from MeOH with Rf value of 0.38.
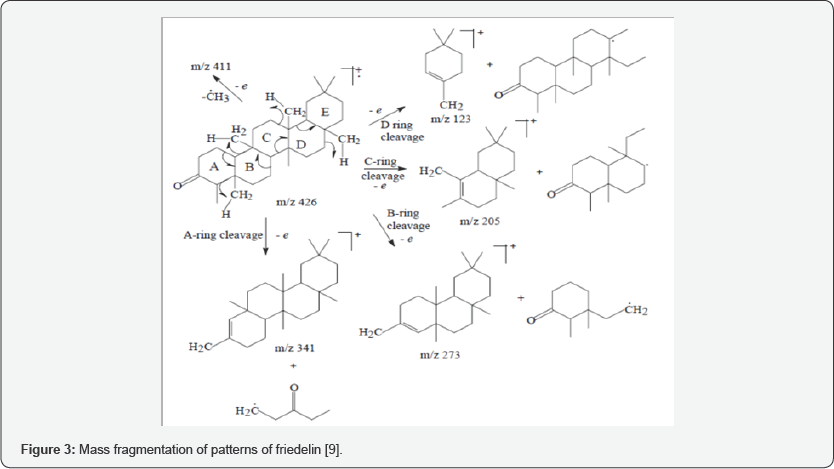
IR (KBr disc): cm'1 3432, 2930, 2870, 1714, 1460, 1388, 1188, 1110, 1074. EIMS m/z (% intensity): 426 ([M]+, 9.74), 411 ([M-CH3]+, 6.80), 302 (9.54), 273 (16.91), 246 (16.40), 231 (15.74), 218 (17.93), 205 (21.44), 191 (18.71), 179 (19.3), 163 (7.67), 149 (18.68), 137 (27.57), 125 (46.19), 123 (57.94), 109 (65.51), 95 (80.07), 81 (66.62). 1H-NMR (600 MHz, CDCl3): 5 0.65 (s, 3H, H- 24), 0.80 (s, 3H, H-25), 0.81 (d, 3H, H-23), 0.88 (s, 3H, H-30), 0.90 (m, 1H, (H-22b), 0.93 (s, 3H, H-29), 0.94 (s, 3H, H-26), 0.98 (s, 3H, H-27), 1.11 (s, 3H, H-28), 1.14 (m, H-19b), 1.19 (m, 1H, H-11b), 1.31 (dd, 1H, H-19a), 1.21 (m, H-6b), 1.24 (m, H-12b), 1.27 (m, H- 15b), 1.28 (m, H-16b), 1.35 (m, H-7b), 1.31 (m, H-12a), 1.35 (m, H-8), 1.37 (m, H-21b), 1.38 (m, H-11a), 1.41 (m, H-22a), 1.42 (m, H- 21a), 1.45 (m, H-7a), 1.48 (m, H-10), 1.49 (m, H-15a), 1.50 (m, H-16a), 1.51 (m, H-18), 1.65 (m, 1H, H-1b), 1.66 (m, H-6a), 1.90 (m, 1H, H-1a), 2.18 (q, 1H, H-4), 2.22 (m, 1H, H-2b), 2.32 (qd, 1H, H-2a). 13C-NMR (150 MHz, CDCl3): 5 6.7 (C-23), 14.6 (C-24), 17.9 (C-25), 18.5 (C-7), 18.6 (C-27), 20.1 (C-26), 22.2 (C-1), 28.1 (C-20), 32.1 (C-17), 30.4 (C-12), 31.7 (C-29), 31.9 (C-28), 29.6 (C-15), 32.7 (C-21), 34.9 (C-30), 35.0 (C-19), 35.5 (C-11), 35.3 (C-16), 35.9 (C- 9), 39.2 (C-14), 39.1 (C-22), 40.9 (C-13), 41.2(C-6), 41.4 (C-2), 42.7 (C-5), 42.0 (C-18), 53.0 (C-8), 58.1 (C-4), 59.5 (C-10), 217.2 (C-3).
d. Minimum Inhibitory Concentration of Friedelin: A concentration of 10 mg/ml of friedelin was prepared and dissolved in acetone in a vial. It was then put in the shaker until all the extract was properly dissolved in acetone. 100 μl of distilled water was added to the 96-well microplates using a multichannel micropipette. Friedelin (100 μl) was added to the first well of the column and serially diluted by two-fold. The fresh bacterial culture was prepared from an overnight culture and diluted with fresh MH broth (1:100). The bacterial culture (100 μl) was added to the test sample in each well of the microtitre plate. The organism and the extract mixtures were incubated for 16 h at 37 oC and after 40 μl of 0.2 mg/ml iodonitrotetrazolium (INT; Sigma) solution was added to each well [3].
Results and Discussion



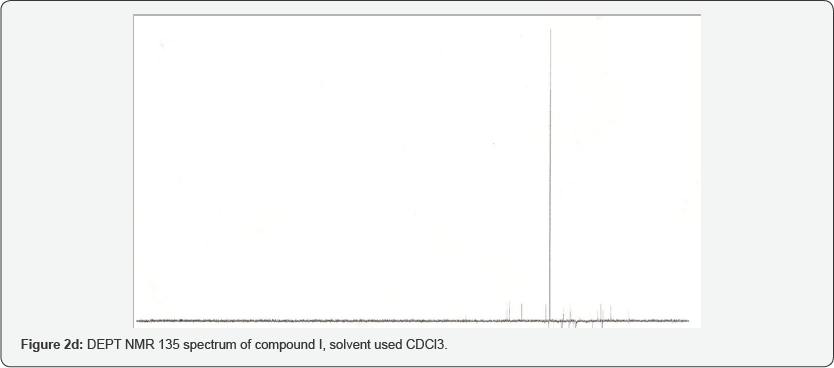


The assignment of the isolated compound (Figure 2a-2g) was based on the chemical shifts obtained from 1D (1H and 13C NMR) and 2D (HMBC, COSY and NOESY) NMR spectral data (Table 1) which indicated the presence of 30 carbons which are typical of triterpenoid of friedelane skeleton and were in line with the reported values of friedelin. The EI mass spectrum gave the molecular ion peak at m/z 426, which correspond to the molecular formula C30H50O (Figure 3). The IR spectrum showed an intense band at 1714 cm-1 which is typical with a six membered ring ketone. The molecular formula was corroborated by the NMR data. The C NMR spectrum showed 30 carbon signals, with characteristic signals for saturated ketone group (5c = 217.21). The DEPT spectrum suggested the presence of a ketone carbonyl at 5 213.1, four sp3 methines eight methyls, eleven sp3 methylenes, and six quarternary sp carbons [3,4].
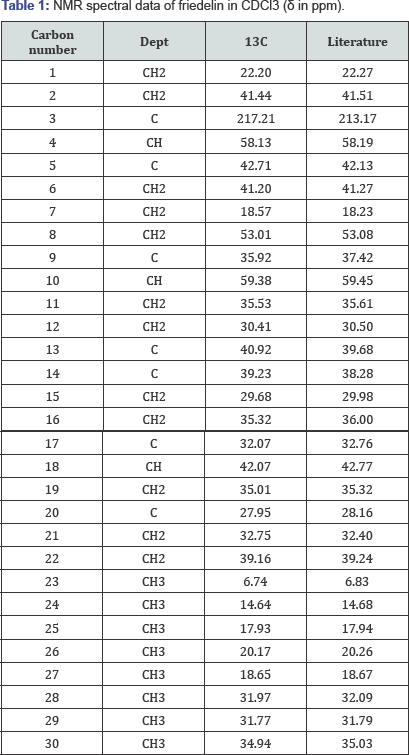
Since the molecular formula indicated six unit of saturation, the compound complied with a pentacyclic triterpene with a ketone group. The presence of signals due to one secondary and seven quarternary methyls in the 1H-NMR spectrum confirmed the friedelane skeleton. The presence of cross correlation between a doublet methyl signal at 5 0.81 and a quartet methine signal at 5 2.18 in the COSY spectrum suggest the presence of a secondary methyl group. The complete chemical shift assignments and literature values are listed in (Table 1). The isolated compound was investigated for antibacterial activity against Staphylococcus aureus, Escherichia coli, Enterococcus faecalis and Pseudomonas aeruginosa as summarized in (Table 2). The antibacterial activity of the isolated compound was performed and it was found to be active against Sa (0.32 μg/ml) and Pa (0.32 &3956;g/ml) [2]. This indicates that friedelin has less antibacterial activity as compared to the positive control which is Gentamycin (Table 2) for all the tested pathogens. Because friedelin is a terpenoidal derivative, it might be suggested that the terpenoidal moiety is important for antibacterial activity.

Conclusion: In conclusion, Friedelin which is reported for the first time from Combretum erythrophyllum was successfully isolated and characterized. Since friedelin showed activity against selected bacterial pathogens, it can be concluded that it is one of the important chemical constituent responsible for the antibacterial activity of the leaves of Combretum erythrophyllum.
Keywords
Keywords: Combretum Erythrophyllum; Friedelin; Antibacterial Activity.
References
- Suffness M, Douros J (1979) Drugs of plant origin. Methods in Cancer Research 26: 73-126.
- Eloff JN (2004) Quantifying the bioactivity of plant extracts during screening and bioassay-guided fractionation. Phytomedicine 11(4): 370-371.
- Eloff JN (1998) A sensitive and quick method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica
- Eloff JN (1999) The antibacterial activity of 27 Southern African members of the Combretaceae. South African Journal of Science 95(3): 148-1S2.
- Schmidt E, Lötter M, McCleland W (2002) Trees and shrubs of Mpumalanga and Kruger National Park.
- Eloff JN, Famakin JO, Katerere DR (2005) Isolation of an antibacterial stilbene from Combretum woodii (Combretaceae) leaves. African Journal of Biotechnology 4(10): 1166-1171.
- Eloff JN, Katerere DR, McGAW LJ (2008) The biological activity and chemistry of the Southern African Combretaceae. Journal of Ethnopharmacology 119(3): 686-699.
- Martini ND, Katerere DR, Eloff JN (2004) Biological activity of five antibacterial Flavonoids isolated from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol 93(2-3): 207-212.
- Susidarti RA, Rahmani M, Ali AM, Sukari MA, HBM Ismail (2014) Friedelin from kelat merah (Eugenia chlorantha Duthie.






























