Synthesis, Characterization & Biological Evaluation of Mutual Prodrugs of Some Selected NSAIDs
Sharma N*
K N Modi Institute of Pharmaceutical Educational & Research, India
Submission: October 31, 2017; Published: November 21, 2017
*Corresponding author: Sharma N, K N Modi Institute of Pharmaceutical Educational & Research, India, Email: nidhi.shar27@gmail.com
How to cite this article: Sharma N. TSynthesis, Characterization & Biological Evaluation of Mutual Prodrugs of Some Selected NSAIDs. Organic & Medicinal Chem IJ. 2017; 4(3): 555639. DOI: 10.19080/OMCIJ.2017.04.555639
Abstract
Non-Steroidal Anti-inflammatory Drugs (NSAIDs) are most widely used drugs because of their Analgesics, Anti-inflammatory & Antipyretic action. Along with these actions it also shows some gastrointestinal & ulcerogenic side effects. To overcoming these side effects it is essential to attach one other moiety with NSAIDs. Mutual prodrugs is a type of prodrug in which two pharmacologically active drugs attached to each other in such a way that each drug act as a promoiety/Carrier for others. In this way the action may be synergistic if they have same biological action or additional if they have different pharmacological action.
Methods for studying antimicrobial activity: Biological evaluation involves testing of the microbial susceptibility to chemotherapeutic agents. Determination of antimicrobial effectiveness against pathogens is essential for proper therapy. Testing can show the efficacy of the antibiotic against pathogen and give an estimate of proper therapeutic dose. The idea of the effectiveness of a chemotherapeutic agent against a specific pathogen can be obtained from the Minimum Inhibitory Concentration (MIC). The MIC is the lowest concentration of a drug that can prevent growth of a specific pathogen (Indian Pharmacopoeia, 1996). Antimicrobial activity is determined based on the in-vitro activity in pure cultures. In-vitro susceptibility tests are done by the following methods:
a. Tube dilution method
b. Agar diffusion method
In this technique petridishes of agar will be prepared by pouring method. The agar will be inoculated with microorganisms. In the agar dilution method different antibiotic concentrations will be incorporated in to an agar medium for both aerobes and anaerobes. The plates are incubated at a temperature of 37 °C for 24 hours. The antimicrobial substance diffuses through the agar and produces a clear zone of inhibition. The diameter of this zone can be measured and an estimation of the degree of activity of the antimicrobial substance can be obtained. Several factors can affect the size of the zone of antibacterial activity. These include: the depth of the medium used, the choice of the medium, the size of the inoculums, the diffusion rate of a particular antibiotic and the last factor in particular has resulted in unfortunate misinterpretation of results. Some laboratories used single or double disk methods. In the single disk method, use one disk of either a high or low antibiotic concentration.
Determining the relative sensitivity of the organism to the drug requires interpretations of zone sizes. With double disk method, the interpretation is simpler. Here both high and low strength disks will apply for each antibiotic to be tested. The organism is reported as being sensitive if a clear zone appears around both disks. If the zone appears around the high concentration alone, the organism is called moderately susceptible. If zones are lacking in both the disks, the organism is considered resistant to drug.
Experimental procedure for Antimicrobial activity
a. Material and methods: The microbiological testing of the synthesized compounds was done by agar well diffusion method.
b. Standard drug: Ciprofloxacin
c. Media: Nutrient agar media were used for the purpose, which contain the constituents (Table 1).
Test strains taken for the determination of the activity:
i. Gram +ve bacterial strains: Bacillus pumerus, Staphylococcus aureus
ii. Gram -ve bacterial strains: Escherichia coli, Klebsiella pneumonia
Agar diffusion method was used for the determination.
Antifungal Activity: An antifungal substance is used to prevent the growth of fungs or to kill the fungus. For determination of activity Agar Diffusion Method was followed.
Test Strain taken for the determination of activity:
a. Candida albicans, Aspergillus nigar
b. Standard drug: Fluconazole.
Antioxidant Activity: An antioxidant is a molecule that inhibits oxidation of other molecules. Oxidation is a chemical reaction that can produce free radicals, leading to chain reactions that may damage cells. The main characteristic of an antioxidant is its ability to trap free radicals. Highly reactive free radicals and oxygen species are present in biological systems from a wide variety of sources. Antioxidant compounds like phenolic acids, polyphenols and flavonoids scavenge free radicals such as peroxide, hydro peroxide or lipid peroxyl and thus inhibit the oxidative mechanisms that lead to degenerative diseases.
Abbreviations: NSAIDs: Non-Steroidal Anti-inflammatory Drugs; MIC: Minimum Inhibitory Concentration; ROS: Reactive Oxygen Species; COXs: Cyclooxygenases
Introduction
Mutual prodrug is a type of prodrug in which two pharmacologically active drugs attached to each other in such a way that each drug act as apromoiety for other. In this way the action may be synergistic if they have same action or additional if they have different pharmacological action. The mutual prodrug concept has shown its marked therapeutic activity with minor undesirable properties and in those active compounds that suffer from severe limitations, like lack of site specificity, poor bioavailability or lack of particular activity. Now a day, several categories of drugs like Anticancer, cardiovascular, antiviral, antipsychotic and anti-inflammatory are utilizing the concept of mutual prodrug designing for their better effect [1].
Mutual Prodrugs can be classifieds into following categories:
i. Carrier- linked mutual prodrugs.
ii. Bipartate carrier linked mutual prodrugs.
iii. Triparte carrier linked mutual prodrugs.
iv. Bio- precursor mutual prodrugs or chemical activation prodrug.
In carrier linked prodrugs, Synergistic drugs act as a carrier Bipartate mutual prodrugs is having a pharmacologically active carrier drug which is directly attached to parent drug where as in case of tripartate mutual prodrugs, the carrier drug is not linked directly to the parent drug but instead through a linker so it allows for decreased steric hindrance during enzymatic cleavage that may occur with bipartite prodrugs. Here Carrier drug is enzymatically cleaved from linker in first step then linker is spontaneously cleaved from parent Drug. Bio precursor mutual prodrugs produce their effects after in vivo chemical modification of their inactive form. Bio precursor prodrugs rely on oxidative or reductive activation reactions unlike the hydrolytic activation of carrier-linked prodrugs [2].
In this paper, we have reviewed the mechanism of formation of mutual prodrugs by using some selected NSAIDs. Non Steroidal Anti inflammatory drugs (NSAIDs) are most widely used drugs because of their anti-inflammatory, analgesics & antipyretic action but along with these it also contain few gastrointestinal & ulcerogenic side effects [3,4]. After many research it was concluded that these ulcerogenic side effects were caused due to the formation of reactive oxygen species (ROS). These observations indicate it must be essential to use an antioxidant to prevent NSAIDs induced gastric ulcer. These antioxidants attached with NSAIDs by glycolic acid spacer [5].
As we go through the mechanism of action of NSAIDs which is related to their ability to inhibit the activity of the enzyme cyclooxygenases (COXs) enzyme that involved in the biosynthesis of prostaglandins specifically prostaglandin H (PGH2) [6]. It is now well known that COX exists in two isoforms, namely COX-I and COX-II, which are regulated differently [7]. COX-I is constitutively expressed in stomach to provide cyto protection in the GIT [8]. COX-II is inducible and plays a major role in prostaglandin biosynthesis in inflammatory cells [9]. Since most of the NSAIDs used clinically inhibit both isoforms, long term use of these agents results in gastric ulcer and there is enough evidence that inhibition of COX-I rather than that of COX-II underlies gastric ulcer formation [10]. NSAIDs cause a dual irruption on the GI tract: the acidic molecules directly irritate the gastric mucosa, and inhibition of COX-1 that reduce the levels of protective prostaglandins [11]. In short Inhibition of prostaglandin synthesis in the GI tract causes increased gastric acid secretion, bicarbonate secretion, mucus secretion and diminished tropic effects on epithelial mucosa [12]. To overcoming the drawbacks associated with NSAIDs it must be essential to temporarily mask the acidic group of NSAIDs [13].
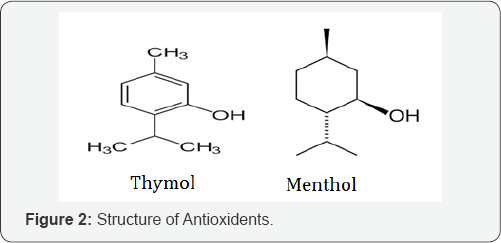
According to the theory, the mutual prodrugs of NSAIDs were synthesized by coupling of NSAIDs (Aceclofenac, Diclofenac) (Figure 1) with Antioxidants (Thymol, Menthol) (Figure 2). In this process the NSAIDs conjugated with antioxidants by Amino acid spacer [14]. These types of prodrugs are known as mutual prodrugs because in this both of the drugs which get couple together have their own pharmacological activity. Antioxidants have their additional benefits as they inhibit the formation of reactive oxygen species (ROS) which is responsible for Gastric ulceration [15]. Non- Steroidal Anti- inflammatory Drugs (NSAIDs) are most widely used drugs. They can be classified in different categories. But in this article the study focused on two anti- inflammatory drugs viz. Diclofenac & Aceclofenac which belongs to different categories of NSAIDs.
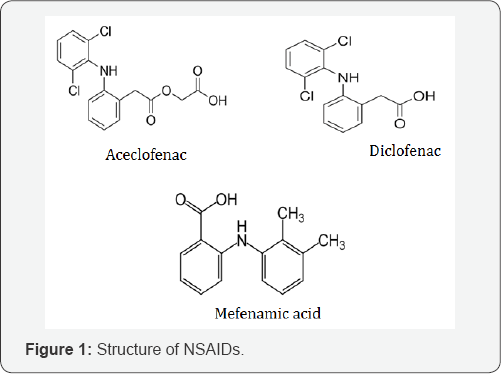
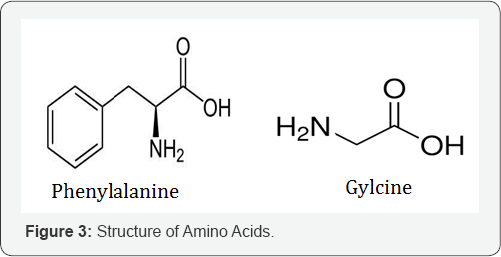
Aceclofenac, Diclofenac are the aryl-acetic acid NSAIDs and Mephenamic acid are the anthranilic acid NSAIDs with good analgesics and antipyretic properties. Diclofenac, is an antiinflammatory drug, similar in efficacy to naproxen. It inhibit PG synthesis is somewhat similar to COX-2 selective. Aceclofenac is somewhat COX-2 selective congener of diclofenac having similar properties. Mephenamic acid is an analgesic, antipyretic and weaker anti- inflammatory drug, which inhibit COX as well as antagonizes certain action of PGs [16] (Figure 3).
Pharmacological Activity
a. Anti- inflammatory: Anti- inflammatory screening of the synthesized compounds was performed by Winter et al in 1962. This activity was performed by following the carrageenan induced paw oedema method in albino rats (120-160g), in this method Diclofenac Sod. (10mg/kg) was used as standard drug for the comparison. Drug and test compound were given orally by preparing suspension in 1% CMC. Injection of carrageenan was done by preparing fresh aqueous suspension (1%w/v, 0.1ml). The suspension was injected in right hind paw of each rat. Mercury displacement (plethysmograph) equipment was used for this method.
b. Purpose and rationale: This method was most commonly used among other methods because this technique was based on the ability of phlogistic agents to inhibit the oedema produced in hind paw of rat after injection. Many phlogistic agents have been used, such as brewer's yeast formaldehyde, dextrane, egg albumin, kaolin, aerosol, sulphated polysaccharides like carrageenan or naphthoylheparamine. The effects can be measured before and after application of irritant (phlogistic agents). Some irritant induce only a short lasting inflammation whereas other irritants cause the paw odema to contribute over more than 24h.
i. Procedure
The animals were divided into groups, 6 rats in each group. One group of animals allotted to control and one group for standard drug (diclofenac sodium). Rest of the groups was allotted to the test compound. Diclofenac sodium and test compound were given orally by preparing with 1% CMC suspension to groups (standard, control and test compound respectively). After 30 min, 0.1mL of 1% freshly prepared carrageenan suspension in 0.9%NaCl solution was injected subcutaneously into the planter aponeurosis of the right hind paw and volume was measured. The foot volume was measured again after 2h and 4h, the mean increase in the pawn volume in each group was calculated. The paw volume was measured by a water plethysmometer apparatus. The difference in volume given the amount of oedema developed.
The percent inhibition value calculated by formula given below
% anti - inflammatory activity = [1 - Dt / Dc ]* 100
Dt and Dc are paw volumes of oedema is tested and control groups respectively
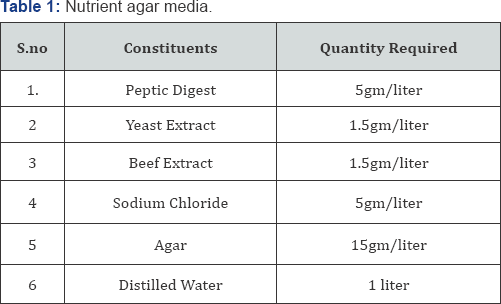
Table 1 anti-inflammatory effect of synthesizes compounds on carrageenan-induced paw edema in rats (10mg/kg), using diclofenac sodium as standard drug (10mg/kg).
Antimicrobial Activity
Antimicrobial drugs have the greatest contribution to the therapeutics. Antimicrobial agents are agents that can inhibit or kill the growth of bacteria such as heat or radiation or a chemical. Antimicrobial drugs can be classified into two categories like Antibacterial drugs & Antifungal drugs. Paul Ehrlich was the scientist who developed firstly the concept of chemotherapy to treat the microbial diseases. He predicted that there are some chemotherapeutic agents that would destroy the pathogens without harming the host. Scientist Pasteur confirmed the fact of antibiotics in 1877; he concluded that airborne bacteria inhibited the development of Anthrax bacilli in urine. Sulpha drugs came into importance in the late 1930.
Alexander Fleming was the scientist who discovered the first antibiotic penicillin in 1929; its first clinical trials were completed in 1940. The modern period of chemotherapy was lightened by Domagk in 1935 by demonstrating the therapeutic effect of Prontosil a sulfonamide dye in pyrogenic infection. But soon it was realized that the active moiety was p-amino benzene sulphonamide, and the dye was not essential. Sulfapyridine was the first sulfonamide. In 1940 Waksman and his colleagues undertook a regular search of actinomycetes as a source of antibiotics and discovered Streptomycin in 1944. This group of soil microbes proved to be a riches house of antibiotics and soon Tetracyclines, Chloramphenicol, Erythromycin and many others followed.
In the past 40 years, importance has shifted from searching new antibiotic producing antibiotics to developing semi synthetic derivatives of older antibiotics with more attractive properties or conflicting spectrum activity. Antimicrobial drugs play an important role in the treatment of many infectious diseases. However repeated and irrational use of some antibiotics results in resistance i.e. ineffectiveness of drug against the microorganisms. In the recent past, the emergence of drug resistance to antibiotics is more. This situation stimulated us to prepare new series of antimicrobials. Drugs are available now with a wide spectrum of activity. However the terms narrow spectrum and broad spectrum are still applied.
Antibacterial drugs
Antibacterial drugs are used to prevent or treat specific bacterial disease without affecting the host. Antibacterial drugs are used in relatively low concentration in or on the bodies of organisms. They can be categorized into three categories viz. Antibacterial drugs, Disinfectant & Antiseptics. Antiseptics & Disinfectants are non-specific with respect to their targets that mean they can kill or inhibit a variety of microbes. Antiseptics drugs are used topically in or on living tissues, differentially Disinfectants are used on objects or in water.
Mechanism of action
They exert their action either by direct killing microorganisms or by inhibiting their growth. Agents like Penicillin, Cephalosporins, and Vancomycin show their action by inhibiting the cell wall synthesis. Polypeptides and polyenes cause leakage from cell membrane. Tetracyclines, Chloramphenicol, Erythromycin etc show their action by inhibiting protein synthesis. Fluroquinolones inhibit DNA gyrase enzyme. Rifampacin and metronidazole interfere with DNA function. Sulfonamides interfere with intermediary metabolism.
References
- Wermuth CG, Ganellin CR, Lindberg P, Mitscher LA (1998) Glossary of terms used in Medicinal Chemistry. Pure and Applied Chemistry 70(5): 1129-1143.
- Vigroux A, Bergon M, Zedde C (1995) Cyclization-Activated Prodrugs: N-(Substituted 2-Hydroxyphenyl and 2-Hydroxypropyl) Carbamates based on Ring-Opened Derivatives of Active Benzoxazolones and Oxazolidinonesasmutual Prodrugs of Acetaminophen. Journal of Medicinal Chemistry 38(20): 3983-3994.
- Laine L (2004) Proton pumps inhibitor co-therapy with nonsteroidal anti-inflammatory drugs-nice or necessary? Rev Gastroenterol Disord 4(suppl 4): S33-S41.
- Scheiman JM (2005) Nonsteroidal anti-inflammatory drugs, aspirin, and gastrointestinal prophylaxis: an ounce of prevention. Rev Gastroenterol Disord 5(suppl 2): S39-S49.
- Singh G, Triadafilopoulos G (1999) Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol 56:18-24.
- Ashutosh Kar (2007) Medicinal Chemistry (4th edn). New Age International Publishers, India pp. 522-535.
- Roche VF (2009) A Receptor-Grounded Approach to Teaching Nonsteroidal Anti-inflammatory Drug Chemistry and Structure-Activity Relationships. American Journal of Pharmaceutical Education 73(8): 143.
- Katzung BG (2004) Basic and clinical pharmacology. McGraw-Hill, New York pp. 298.
- Hardman JG, Limbird LE, Molinoff PB (2001) Goodman and Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill, New York pp. 689.
- Hawkey CJ (1999) COX-2 inhibitors. Lancet 353(9149): 307-314.
- Ajai kumar K, Asheef M, Babu B, Padikkala J (2005) The inhibition of gastric mucosal injury by Punicagranatum L (pomegranate) methanolicextract. Journal of Ethnopharmacology 96(1-2): 171-176.
- Simone Rossi (2006) Australian medicines handbook.
- Parmeshwari K Halen, Prashant R Murumkar, Rajani Giridhar, Mange Ram Yadav (2009) Prodrug Designing of NSAIDs. Mini-Reviews in Medicinal Chemistry 9(1): 124-139.
- Chen HY, Lin YC, Hsieh CL (2007) Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem 104(4): 1418-1424.
- Repetto MG, Llesuy SF (2002) Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Br J Med Biol Res 35(5): 523-534.
- Tripathi KD, Essential of Medical Pharmacology.






























