Chemical Constituents from the Leaves of Machilus Bombycina King Ex Hook.f.
Mohammed Ali1*, Kamaruz Zaman1,2, Showkat Rassol Mir1 and Shahnaz Sultana1,3
1Phytochemistry Research Laboratory, School of Pharmaceutical Education and Research, Jamia Hamdard, India
2Department of Pharmaceutical Sciences, Dibrugarh University, India
3College of Pharmacy, Jazan University, Saudi Arabia
Submission: October 28, 2017; Published: November 10, 2017
*Corresponding author: Mohammed Ali, Phytochemistry Research Laboratory, School of Pharmaceutical Education and Research, Jamia Hamdard, India, Email: maliphyto@gmail.com
How to cite this article: Mohammed A, Kamaruz Z, Showkat R Shahnaz S. Chemical Constituents from the Leaves of Machilus Bombycina King Ex Hook.f.. Organic & Medicinal Chem IJ. 2017; 4(3): 555636. DOI: 10.19080/OMCIJ.2017.03.555636
Abstract
Machilus bombycina King ex Hook. f. (Lauraceae) is found in China and India. Its leaves are used to cure pimples and rheumatism. Column chromatography of a methanolic extract of the leaves of M. bombycina afforded 8, 21-dihydroxylanost-5, 25-dien-3-olyl behenate (1) and 5,7-dihydroxy-3', 4'-dimethoxyflavanone-7-O-a-D-glucopyranosyl-(6''^1''')-a-D-rhamnopyranoside (2) as the new phytoconstituents.
a.Results and Discussion: Compound 1, named 8, 21-dihydroxylanostdien-3-olyl behenate, responded positively to Liebermann-Burchardt test for triterpenoids and showed IR absorption bands for hydroxyl groups (3510, 3416 cm-1), ester function (1721 cm-1), unsaturation (1640 cm-1) and long aliphatic chain (727 cm-1). On the basis of mass and 13CNMR spectra the molecular ion peak of 1 was determined at m/z 780 consistent with a molecular formula of a lanostanyl ester, C52H92O4. The ion fragments arising at m/z 457 [C1'-O fission, C30H49O3]+, 339[M-457, CH3(CH2)20COO]+, 440 [457-OH]+, 127 [C17-C20 fission, C8H15O side chain]+ and 313 [440-side chain, C30H48O3]+ indicated that the attachment of a behanate group linked to the lanostandiene unit possessing a C8 side chain with a hydroxyl group and a vinylic bond. The 1H NMR spectrum of 1 showed a one-proton multiplet at 6 5.37 assigned to vinylic H-5, two one-proton signals at 6 4.92 and 4.90 due to exocyclic methylene H2-26 protons, a one-proton double doublet at 6 4.38 with coupling interactions of 5.3 and 9.5 Hz attributed to oxymethine H-3a proton, a two-proton doublet at 6 3.21 (J=6.5 Hz) accounted to hydroxymethylene H2-21 protons, six three-proton singlets between 6 1.92-1.01 accommodated to tertiary C-18 to C-30 methyl protons and a three-proton triplet at 6 0.83 (J=6.1 Hz) ascribed to primary C-22' methyl protons.
The other methylene and methine protons resonated as multiplets from 6 2.72 to 1.55 and as a broad signal at 6 1.28 (36 H). The 13C NMR spectrum of 1 showed signals for ester carbon at 6 173.16 (C-1'), vinylic carbons between 6 142.97-116.13, oxymethine carbon at 6 79.30 (C-3), hydroxymethylene carbon at 6 63.06 (C-21), and methyl carbons from 6 21.09 to 14.04. The 1H and 13C NMR spectral data of the triterpenic unit of 1 were compared with the reported data of lanostene-type triterpenoids [1,2]. Acid hydrolysis of 1 yielded behenic acid, m. p. 79 - 80 °C. On the basis of above discussion the structure of 1 has been elucidated as 8,21-dihydroxylanost-5,25-dien-3-olyl behenate, a new lanostane type- triterpenic ester.
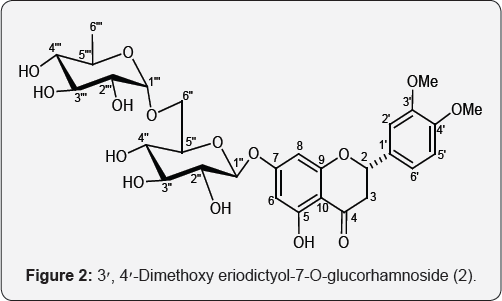
Compound 2, named 3',4'-dimethoxyeriodictyol 7-O-glucorhamnoside, responded positively to phenolic and glycosidic tests and had UV absorption maxima at 287 and 328 typical for a flavanone derivative. It showed a bathochromic shift of 40 nm on addition of AlCl3 and AlCl3/HCl suggesting the presence of a chelated hydroxyl function at C-5. Its IR spectrum disclosed a characteristic absorption for the conjugated carbonyl group (1668 cm-1) and hydroxyl groups (3510, 3418, 3217 cm-1). On the basis of its mass and 13C NMR spectra the molecular ion peak of 2 was determined at m/z 624 consistent with a molecular formula of a flavonoid diglycoside, C29H36O15. The important ion peaks generated at m/z 609 [M - Me]+, 581 [609 - CO]+, 147 [C/'' - O fission, C6H11O4]+, 477 [M - 147]+, 326 [C7 - O fission, C^H^O^ , 298 [M - 326, C17H14O5]+ and 315 [C1'' - O fission, C17H15O6]+ supported that a dihexoside unit was linked with the ring A of the flavanone. The ion fragments produced at m/z 300 [315-Me]+, 253 [315 - 2 x OMe]+, 164 [C34-C2O fission]+ and 150 [C^-C^ fission]+ indicated the presence of two methoxy groups in ring B and methylene protons at C-3.
The 1H NMR spectrum of 2 showed an ABX system of resonances as one-proton double doublets at 6 5.56 (J = 3.2, 12.9 Hz, H-2), 3.19 ( J = 2.8, 17.0 Hz, H2-3a) and 2.81 (J = 3.2, 12.9 Hz, H2-3b) characteristic of oxymethine H-2 and methylene H2-2 eq and H2-2 ax, respectively, of a flavanone moiety?. Four one-proton doublets at 6 7.01 (J = 2.8 Hz), 6.21 (J = 2.0 Hz), 6.17 (J = 2.0 Hz), 6.96 (2 = 8.4 Hz), and a one-proton double doublet at 6 6.99 (J = 2.8, 8.4 Hz) were ascribed to meta-coupled H-2', H-8 and H-6, ortho-coupled H-5' and meta-ortho-coupled H-6' protons, respectively. Two one-proton doublets at 6 5.48 (J = 4.8 Hz) and 5.27 (J = 5.3 Hz), a three-proton doublet at 6 1.21 (J = 7.2 Hz) and two three- proton broad signals at 6 3.82 and 3.44 were accounted correspondingly to anomeric H-1'' and H-1''', secondary methyl H3-2''' of rhamnose unit and two methoxy protons.
The 13C NMR spectrum of 2 displayed 29 signals including a carbonyl carbon of a flavanone at 6 197.03 (C-4), oxymethine at 6 78.41 (C-2), methylene carbon of the flavanone at 6 42.23 (C-3), methoxy carbons at 6 55.60 and 58.57, anomeric carbons at 6 100.56 (C-1'') and 96.31 (C- 1'''), methyl carbon at 6 17.81 (C-6''') and other sugar carbons between 6 76.20-65.97. The DEPT spectrum of 2 exhibited the presence of three methyl, two methylene, seven methine and eight quaternary carbons. The existence of the oxymethylene H2-6'' proton signal in the deshielded region as a doublet at 6 3.23 (J = 8.4 Hz, H2-6'') and its C-6'' carbon signal at 6 65.97 (C-6'') suggested (6''^!'") linkage of the sugar moieties.
Acid hydrolysis of 2 yielded dimethoxyeriodictyol, D-glucose, Rf 0.18 (n-butanol-acetic acid-water, 4:1:5) and D-rhamnose, Rf 0.86 (n-butanol- N acetic acid-water, 4:1:1.6). On the basis of these evidences the structure of 2 was formulated as 5,7-dihydroxy-3',4'-dimethoxyflavanone-7-O-a-D- glucopyranosyl-(6''^1''')-a-D-rhamnopyranoside,a new flavanone diglycoside.
Conclusion: Phytochemical investigation of a methanolic extract of the leaves of Machilus bombycina afforded a lanostene-type3-olyl behenate and a flavanone-7-O-a-D-glucopyranosyl (6''^1''')-a-D-rhamnopyranoside. This work has enhanced understanding about the phytoconstituents of these plants. These secondary metabolites can be used as analytical markers for quality control of these herbal drugs.
Keywords: Machilus Bombycina Leaves; Chemical Constituents; Isolation; Characterization
Introduction
Machilus Bombycina King ex Hook. f. (Lauraceae), known as som, is distributed in China, India and other south eastern countries. It is one of the primary food plants of Antheraea assama Westwood, the silkworm that produces muga or golden colour natural silk. Its leaf paste is applied to relieve rheumatism. Juice of the leaves of M. bombycina and Achyrenthus aspera is applied to cure pimples [3]. Chlorogenic acid, phytic acid, tannins, catechol, morin, gallic acid, p-sitosterol and its 3-O-glucoside are reported from the leaves [4]. The plant essential oil was mainly consisted of decanal, 11-dodecenal and dodecanal [5]. The major constituents of the flower oil were caryophyllene oxide, (E)-nerolidol, 11-dodecenal and 11-dodecenoic acid. The fruit oil was composed of the furanoid forms of trans- and cis- linalool oxides [6,7]. The manuscript describes isolation and characterization of one each of triterpenic ester and flavanone glycoside from the leaves of M. bombycina.
Extraction and Isolation
The leaves of M. bombycina (1 kg), procured from Dibrugarh, Assam were coarsely powdered and extracted exhaustively separately with methanol in a Soxhlet apparatus. The extracts were concentrated under reduced pressure to get a dark brown mass (115.6). The dried residue (100 g) was dissolved in minimum amount of methanol and adsorbed on silica gel column grade (60-120 mesh) to prepare a slurry. It was air-dried and chromatographed over silica gel columns loaded in chloroform. The column was eluted with chloroform and chloroform - methanol mixtures to isolate the compounds 1 and 2.
a. 8,21-Dihydroxylanostdien-3-olyl behenate (1):Elution of the column with chloroform-methanol (49:1) produced colorless crystals of 1, yield 163 mg, m. p. 233-235 °C; IR v (KBr): 3510, 3416, 2918, 2854, 1721, 1640, 1461, 1373,max v J1272, 1123, 727 cm-1; ^ NMR (CDCl3): 5 5.37 (1H, m, H-5), 4.92 (1H, s, H2-26a), 4.90 (1H, s, H2-26b), 4.38 (1H, dd, J = 5.3, 9.5 Hz, H-3a), 3.21 (2H, d, J = 6.5 Hz, H2-21), 1.92 (3H, brs, Me-27), 1.21 (3H, s, Me-28), 1.19 (3H, brs, Me-29), 1.16 (3H, brs, Me-19), 1.14 (3H, brs. Me-30), 1.01 (3H, brs, Me-18), 2.72 (2H, m, H2-7), 2.02 -1. 33 (21H, m, 9 x CH2, 3 x CH), 2.35 (2H, t, J = 7.5 Hz, H2-2’), 1.55 (2H, m, H2-3’), 1.28 (36 H, brs, 18 x CH2), 0.83 (3H, t, J = 6.1 Hz, Me-22'); 13C NMR (CDCl3): 5 39.34 (C-1), 25.75 (C-2), 79.30 (C- 3), 42.32 (C-4), 142.97 (C-5), 121.71 (C-6), 28.26 (C-7), 71.81 (C- 8), 50.12 (C-9), 36.79 (C-10), 23.07 (C-11), 29.13 (C-12), 45.42 (C-13), S6.76 (C-14), 33.94 (C-1S), 31.60 (C-16), S9.SS (C-17),11. 79 (C-18), 21.09 (C-19), 30.79 (C-20), 63.06 (C-21), 37.25 (C-22), 24.14 (C-23), 42.83 (C-24), 140.74 (C-2S), 116.13 (C-26),19.40 (C-27), 19.1S (C-28), 19.04 (C-29), 18.79 (C-30), 173.16 (C-1'), 56.05 (C-2'), 47.87 (C-3'), 30.68 (C-4'), 29.72 (C-5'), 29.69 (C-6'), 29.64 (C-7'), 29.45 (C-8'), 29.28 (C-9' to C-14'), 29.13 (C- 15' to C-18'), 27.23 (C-19'), 24.31 (C-20'), 21.11 (C-21'), 14.04 (C-22'); ESI MS m/z (rel. int.): 780 [M]+ (C52H92O4) (16.2), 457 (12.5), 440 (20.5), 339 (32.8), 313 (31.8), 127 (23.6) (Figure 1).

b. 3',4'-Dimethoxyeriodictyol 7-O- glucorhamnoside (2): Elution of column with chloroform-methanol (3:1) furnished yellow crystals of 2, yield 155 mg, m.p.174-176°C, UV Amax (MeOH): 287, 328 nm; IR Ymax (KBr): 3510, 3418, 3217, 2927, 2835, 1668, 1601, 1453, 1405, 1326, 1127, 1076 cm- ^ NMR (DMSO-d6): 5 7.01 (1H, d, J=2.8 Hz, H-2'), 6.99 (1H, dd, J=2.8, 8.4Hz, H-6'), 6.96 (1H, d, J=8.4 Hz, H-5'), 6.21 (1H, d, J=2.0 Hz, H-8), 6.17 (1H, d, J=2.0 Hz, H-6), 5.56 (1H, dd, J=3.2, 12.9 Hz, H-2), 3.19 (1H, dd, J=2.8, 17.0 Hz, H2-3a), 2.81 (1H, dd, J=3.2, 12.9 Hz, H2-3b), 3.82 (3H, brs, OMe), 3.44 (3H, brs, OMe), 5.48 (1H, d, J=4.8 Hz, H-1''), 4.84 (1H, m, H-5''), 4.71 (1H, dd, J=4,8, 6.4 Hz, H-2''), 3.69 (1H, m, H-3''), 3.42 (1H, m, H-4''), 3.23 (2H, d, J=8.4 Hz, H2-6''), 5.27 (1H, d, J=5.3 Hz, H-1'''), 4.79 (1H, m, H-5'''), 4.58 (1H, m, H-2'''), 3.51 (1H, m, H-3'''), 3.35 (1H, m, H-4'''), 1.21 (3H, d, J=7.2 Hz, Me-6'''), 13C NMR (DMSO-d6): 5 78.41 (C-2), 42.23 (C-3), 197.03 (C-4), 162.96 (C-5), 99.14 (C-6), 165.07 (C-7), 95.48 (C-8), 162.50 (C-9), 103.25 (C-10), 130.89 (C-1'), 114.09 (C-2'), 146.35 (C-3'), 147.90 (C-4'), 117.94 (C-5'),111. 98 (C-6'), 55.60 (OMe), 58.57 (OMe), 100.56 (C-1''), 72.01 (C-2''), 70.63 (C-3''), 69.52 (C-4''), 76.20 (C-5''), 65.97 (C-6''), 96.31 (C-1'"), 72.92 (C-2"), 70.22 (C-3'"), 68.29 (C-4'"), 75.44 (C-5'"), 17.81 (C-6'''); ESI MS m/z (rel. int.): 624 [M]+ (C29H36O15) (5.2), 609 (5.7), 581 (12.1), 477 (14.9), 326 (25.8), 315 (11.9), 300 (33.6), 298 (32.1), 253 (38.7), 164 (21.2), 150 (25.5), 147 (22.3), 105 (35.8) (Figure 2).
References
- Ali M (2001) Techniques in Terpenoid Identification, Birla Publications. Delhi pp. 352-360.
- Bagri P, Ali M, Aeri V, Bhowmik M (2016) Isolation and Antidiabetic Activity of new Lanostenoids from the Leaves of Psidium Guajava L. Int J Pharm Pharm Sci 8(9):14-18.
- Quattrocchi U (2012) CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific names, Synonyms and Etymology, CRC, Boca Raton, Florida 5: 51.
- Neog K, Das A, Unni BG, Ahmed GU, Rajan RK (2011) Studies on Secondary metabolites of Som (Persea bombycina Kost), a primary host plant of Muga silkworm (Antheraea assamensis Helfer). International Journal of Pharm Tech Research, India 3 (3): 1441-1447.
- Choudhary SN, Leclercq PE (1995) Essential Oil of Machilus bombycina King from Northeast India. J Essent Oil Res 7(2): 199-201.
- Choudhury SN, Ghosh AC, Choudhury M, Leclercq PA (1997) Constituents of the flowers and fruits oils of Persea gamblei (King ex Hook. f.) Kost from India. J Essent Oil Res 9(2): 177-180.
- Choudhury SN, Vajczikova I (2003) Variation in the essential oil composition of Persea bombycina (King ex Hook.f.) Kost and its effect on muga silkworm (Antheraea Assama Ww)-a new report. Indian J Chem 42B: 641-647.






























