Click Reaction for the Synthesis of Phenylmethylene Hydrations
Masoumeh Mortazi and Farahnaz K Behbahani*
Department of Chemistry, Karaj Branch, Islamic Azad University, Iran
Submission: October 27, 2017; Published: November 10, 2017
*Corresponding author: Farahnaz K Behbahani, Department of Chemistry, Karaj Branch, Islamic Azad University, Karaj, Iran, Email:farahnazkargar@yahoo.com
How to cite this article: Masoumeh M Farahnaz K B. Click Reaction for the Synthesis of Phenylmethylene Hydrations. Organic & Medicinal Chem IJ. 2017; 4(2): 555635. DOI: 10.19080/OMCIJ.2017.03.555635
Abstract
Introduction: A simple method for preparing phenylmethylene hydrations is proposed based on a Knoevenagel Condensation using aromatic aldehydes and hydrations in the presence of ethanol amine in ethanol/water under reflux condition. The synthetic process is interesting and synthesized phenylmethylene hydantoins are new compounds.
Conclusion: In conclusion, we have developed an improved synthetic method for the synthesis of phenylmethylene hydrations in good-to- excellent yields in mild medium. This method has the advantages of good yields, mild reaction conditions, easy work-up, inexpensive reagents and being environmentally friendly over the existing procedures.
Keywords: Phenylmethylene Hydrations; Ethanol Amine; Aldehydes; Synthesis
Introduction
Imidazolidine-2, 4-diones, or hydantoins have been widely used in biological screenings resulting in numerous pharmaceutical applications. Indeed, many derivatives have been recognized as anti-convulsants [1] and antimuscarinics, [2] antiulcer and antiarrythmics, [3] antiviral, antidiabetics, [4] serotonin and fibrinogen receptor antagonists, [5] inhibitors of the glycine binding site of the NMDA receptor, [6] and antagonists of leukocyte cell adhesion acting as allosteric inhibitors of the protein-protein interaction [7]. Moreover, substituted hydantoins are important building blocks for the synthesis of non-natural amino acids both in raceme form by alkaline degradation [8] and in an enantioselective way by enzymatic resolution [9]. For this reason, there is a lot of interest in developing new strategies for a straightforward synthesis of selectively substituted hydantoins both in solution and in solid phase [10]. Also, hydantoins were considered good scaffolds for future design of tyrosine kinase inhibitors [11].
Ethanolamine is commonly called monoethanolamine or MEA in order to be distinguished from diethanolamine (DEA) and triethanolamine (TEA). Ethanolamine is the second-most- abundant head group for phospholipids, a substance found in biological membranes, and is also used in messenger molecules such as palmitoylethanolamide which has an effect on CB1 receptors [12]. MEA is used in aqueous solutions for scrubbing certain acidic gases. It is used as feedstock in the production of detergents, emulsifiers, polishes, pharmaceuticals, corrosion inhibitors, chemical intermediates [13,14]. For example, reacting ethanolamine with ammonia gives the commonly used chelating agent, ethylenediamine: [13] In pharmaceutical formulations, MEA is primarily used for buffering or preparation of emulsions. MEA can be used as pH regulator in cosmetics. Here we interest to develop a novel method for the synthesis of phenylmethylene hydrations using aromatic aldehydes and hydantoine in the presence of ethanolamine in water/ethanol under reflux conditions (Figure 1).

Results and Discussion
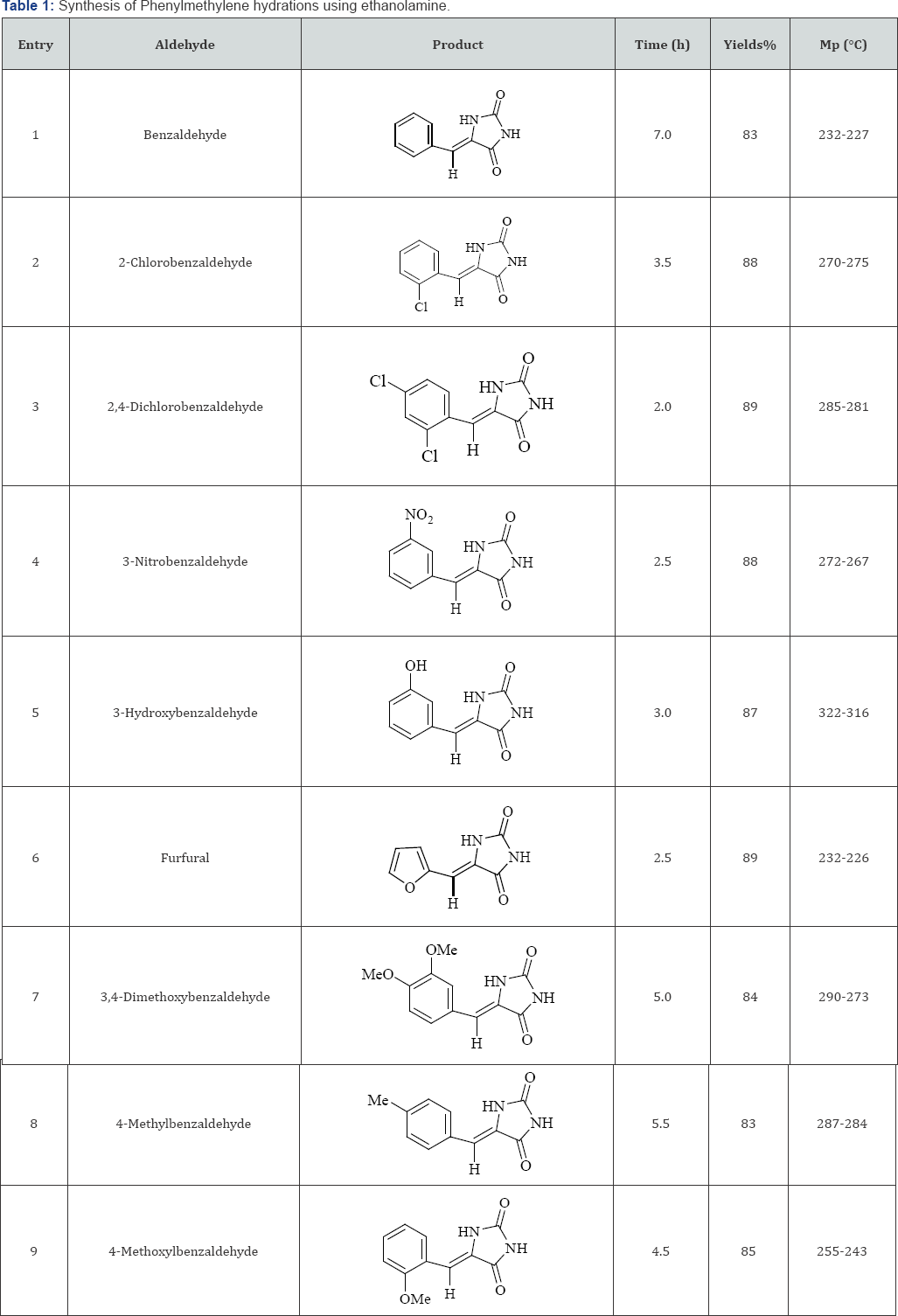
To generalize this reaction to a library synthesis, hydration was treated with various aldehydes using ethanolamine in ethanol/water which successfully yielded the corresponding phenylmethylene hydantoines (Table 1). As shown in Table 1 in all cases, phenylmethylene hydantoines were obtained in good- to-excellent yields. In the present procedure, aromatic aldehyde carrying electron-withdrawing substituents in benzene ring reacted faster than those of possessing electron-donating groups. The longer reaction times were observed for the substrates bearing electron-donating groups (Table 1). The present method was convincingly superior to the reported methods with respect to yield, reaction time, simplicity and safety.
Experimental
a. Preparation of phenyl ethylene hydrations General procedure: Hydration (10 mmol, 1.0 g) was dissolved in 10 mL H2O while heating at 70 °C on oil bath with continuous stirring. After complete dissolution, the pH was adjusted to 7.0 using saturated NaHCO3 solution. The temperature was then raised to 90 °C after the addition of 0.9 mL ethanolamine (0.015 mmol,0.9.mL). A solution of aldehyde (10 mmol in 2-5 mL EtOH) was added drop wise with continuous stirring. The reaction was kept under reflux for approximately 2-7 h. The reaction was monitored by TLC every hour after a yellow or white precipitate is formed. After complete depletion of the starting material, the mixture was cooled and the precipitate was filtered and washed with EtOH/H2O (1:5) before recrystallization from EtOH. Reaction yield range from 80-90%, based on the nature of the used aldehyde.
References
- J C Thenmozhiyal, P T H Wong, W K Chui (2004) Anticonvulsant activity of phenylmethylene hydrations: a structure-activity relationship study. J Med Chem 47(6): 1527-1535.
- (a) C W Bazil and T A Pedley (1998) Advances in the Medical treatment of epilepsy. Annu Rev Med 49: 135-162; (b) M S Luer (1998) Fosphenytoin. Neurol Res 20(2): 178-182.
- (a) M Matsukura, Y Daiku, K Ueda, S Tanaka, T Igarashi, et al. (1992) Synthesis and Ant arrhythmic activity of 2,2-dialkyl-1'-(N-substituted amino alkyl)-spiro-(chroman-4,4'-imidazolidine)-2',5'-diones. Chem Pharm Bull, Tokyo 40(7): 1823-1827; (b) J Knabe, J Baldauf, A Ahlhelm (1997) Racemates and Enantiomers of Basic, Substituted 5-phenylhydantoins, Synthesis and Anti-arrhythmic Action. Pharmazie 52(12): 912-9.
- L Somsa k, L Kova cs, M To th, E O sz, L Szilagyi, et al. (2001) Synthesis of and a comparative study on the inhibition of muscle and liver glycogen phosphorylases by epimeric pairs ofd-gluco- and d-xylopyranosylidene- spiro-(thio)hydantoins and N-(d-glucopyranosyl) amides. J Med Chem 44(17): 2843-2848.
- (a) G P Moloney, A D Robertson, G R Martin, S MacLennan, N Mathews, et al. (1997) A novel series of 2,5-substituted tryptamine derivatives as vascular 5HT1B/1D receptor antagonists. J Med Chem 40(15): 2347-2362; (b) G P Moloney, G R Martin, N Mathews, A Milne, H Hobbs, et al. (1999) Synthesis and serotonergic activity of substituted2, N-benzylcarboxamido-5-(2-ethyl-1-dioxoimidazolidinyl N-dimethyltryptamine derivatives: novel antagonists for the vascular 5-HT(1B)-like receptor. J Med Chem 42(14): 2504-2526.
- M Jansen, H Potschka, C Brandt, W L Oscher, G Dannhardt (2003) Hydantoin-substituted 4,6-dichloroindole-2-carboxylic acids as ligands with high affinity for the glycine binding site of the NMDA receptor. J Med Chem 46(1): 64-73.
- K Last Barney, W Davidson, M Cardozo, L L Frye, C A Grygon, et al. (2001) Binding site elucidation of hydantoin-based antagonists of LFA-1 using multidisciplinary technologies: evidence for the allosteric inhibition of a protein-protein interaction. J Am Chem Soc 123(24): 5643-5650.
- J C Sutherland, G P Hess (2000) Synthesis of fluorinated amino acids. Nat Prod Rep 17(6): 621-631.
- (a) C Syldatk, R M uller, M Siemann, K Krohn, F Wagner (1992) in Biocatalytic Production of Amino Acids and Derivatives, (ed J D Rozzell and F Wagner), Hanser, M unchen p. 75; (b) K Drauz, H Waldmann (1995) Enzyme Catalysis in Organic Synthesis, VCH, Weinheim p. 409.
- Some very recent papers dealing with the synthesis of hydantoins: (a) T Miura, Y Mikano, M Murakami (2011) Org Lett 13: 3560; (b) G Baccolini, C Boga, C Delpivo, G Micheletti (2011) Tetrahedron Lett 52: 1713; (c) O A Attanasi, L De Crescentini, G Favi, S Nicolini, F R Perrulli, et al. (2011) Org Lett 13: 353; (d) I A Hashmi, A Aslam, K Ali Syed, A Vigar uddin, I A Firdous (2010) Synth Commun 40: 2869; (e) M Gao, Y Yang, Y D Wu, C Deng, W M Shu, et al. (2010) Org Lett 12: 4026; (f) S M Dumbris, D J Diaz, L Mc Elwee White (2009) J Org Chem74: 8862.
- Jemal A, Rebecca Siegel R, Ward E, Hao Y, Xu J, et al. (2008) Cancer statistics. CA Cancer J Clin 58(2): 71-96.
- Calignano A, La Rana G, Piomelli D (2001) Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. European Journal of Pharmacology 419 (2-3): 191-8.
- Klaus Weissermel, Hans Jürgen Arpe, Charlet R Lindley, Stephen Hawkins (2003) Oxidation Products of Ethylene. Industrial Organic Chemistry. Wiley VCH pp. 159-161.
- Carrasco F (2009) Ingredients Cosméticos. Diccionario de Ingredientes Cosméticos 4a Ed pp. 306.






























