Facile Synthesis of Some Novel 1, 3, 4-Oxa diazole Derivatives Associated with Pyrimidine Core Unit as a Anti-Microbial Agents
Virupakshi Prabhakar1*, Sreenivasulu Peta1 and Srinivasula Reddy Maddula2
1Faculty of Chemistry, Rajiv Gandhi University of knowledge technologies, India
2Prajna Generics Private Limited, India
Submission: October 10, 2017; Published: November 10, 2017
*Corresponding author: Prabhakar , Dr.Apj Abdul kalam-IIIt Ongole, Rajiv Gandhi university of knowledge technologies, India, E-mail: viruchem765@gmail.com
How to cite this article: Virupakshi P, Sreenivasulu P, Srinivasula R M. Facile Synthesis of Some Novel 1, 3, 4-Oxa diazole Derivatives Associated with Pyrimidine Core Unit as a Anti-Microbial Agents. Organic & Medicinal Chem IJ. 2017; 4(2): 555634. DOI: 10.19080/OMCIJ.2017.03.555634
Abstract
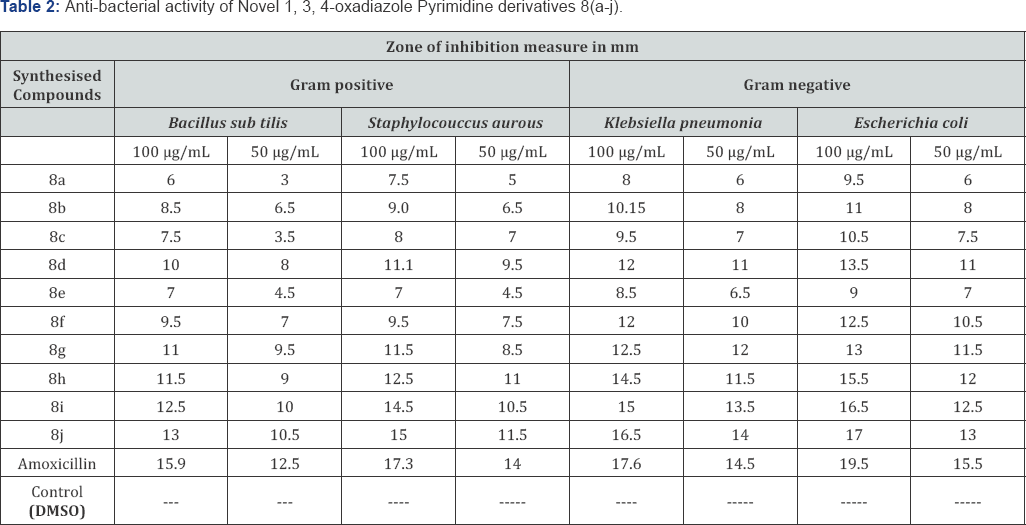
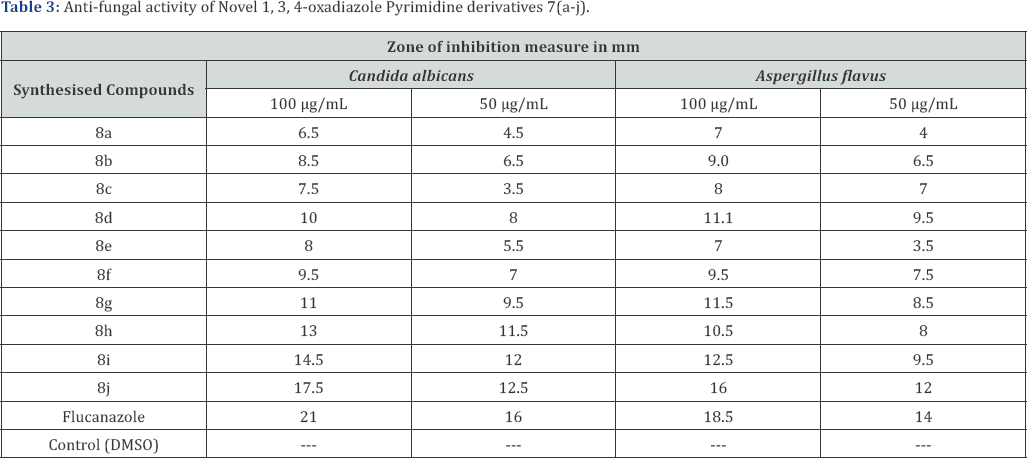
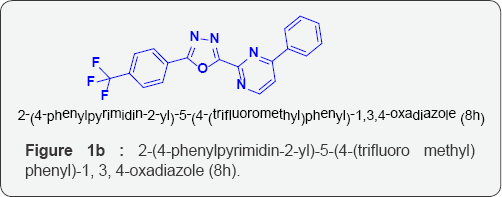
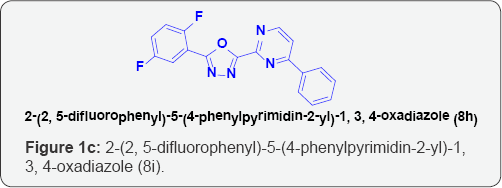
Aim: A new series of 2-(4-phenylpyrimidin-2-yl)-5-p-substituted-1, 3, 4-oxadiazole derivatives (8a-j) were synthesized after refluxing4- phenylpyrimidine-2-carbohydrazide[6] with different aromatic/Heterocyclic carboxylic acids (7 a-j) in the presence of POCl3. The chemical structures of these compounds were confirmed by various physico-chemical methods viz. IR, [1] H-NMR, EI-Mass, [13] C-NMR analysis. Newlysynthesized compounds were screened in vitro for their antimicrobial activity against varieties of gram-positive and gram-negative bacterialstrains and fungi strains. The compounds 8j, 8i and 8h shows highly significant antimicrobial activity as compared to the standard drug.
Materials and Methods: Melting points of the synthesized compounds were determined in Open-glass capillaries using GUNA melting point apparatus and are uncorrected. IR Absorption spectra were recorded in the 4000-400 cm-1 range on a Shimadzu FTIR-8400s Using KBr pellets, [1] H-NMR and [13] C-NMR were recorded on Bruker-NMR, 400 MHz Spectro photometer. EI-Mass spectra were recorded by Agilent-NMR, 400 MHz Spectro photometer. TLC was done on F254 grade silica-60 from SD Fine. Antimicrobial studies were done in the Microbiology Laboratory in S.K. University Anantapuramu.
Results and Discussion
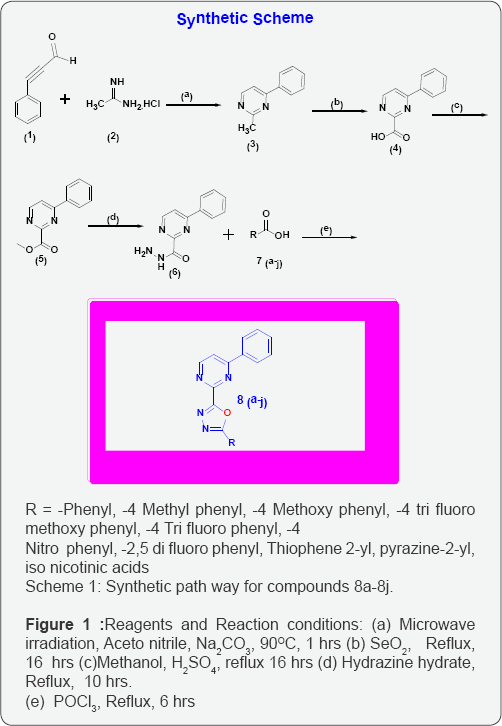
Chemistry: In the present work, 1, 3, 4-oxadiazole derivatives containing Pyrimidine nucleus unit8 (a-j) were synthesized and characterized on the basis of spectral (1) H NMR, (13) C-NMR, EI-Mass and IR) analyses. The synthesized compounds were evaluated for their antimicrobial activity against different bacteria and fungi strains. Synthetic chemistry involves conversion of corresponding aromatic acids to respective Methyl esters (5) and further converted to carbohydrazides (6) by refluxing with hydrazine hydrate. Various Para substituted carboxylic acids 7 (a-j) was refluxed in the presence of POCl3 and obtained a series of derivatives of 1, 3, 4-oxadiazole containing Pyrimidine core unit 8 (a-j).
Biology: Two gram negative and two gram positive bacterial strains were used for the evaluation of the antibacterial screening of the selected synthesized compounds. Ampicilin was used as the standard. Similarly, two fungi strains were used for the antifungal screening of the selected compounds. Flucanazole was used as the standard. The results were illustrated in the (Tables 1-3) respectively. The compounds 8j, 8i and 8h shows highly significant antimicrobial activity as compared to the standard drug. Rest of the compounds showed moderate inhibition on bacteria and fungi strains.
Conclusion: A new series of 2-(4-phenylpyrimidin-2-yl)-5-p-substituted-1, 3, 4-oxadiazole derivatives (8a-j) were synthesized. The synthesized compounds were also moderately active against different bacteria and fungi Strains. Among the selected compounds, 7h and 7i exhibited good antibacterial and antifungal agent in our study. These results indicated that substituted 1, 3,4oxadiazole with Pyrimidine core unit may be useful leads for Anti-microbial drug development in the future.
Keywords: 1, 3, 4-Oxadiazole; Pyrimidines; Cyclisation; Antibacterial and Antifungal activity
Introduction
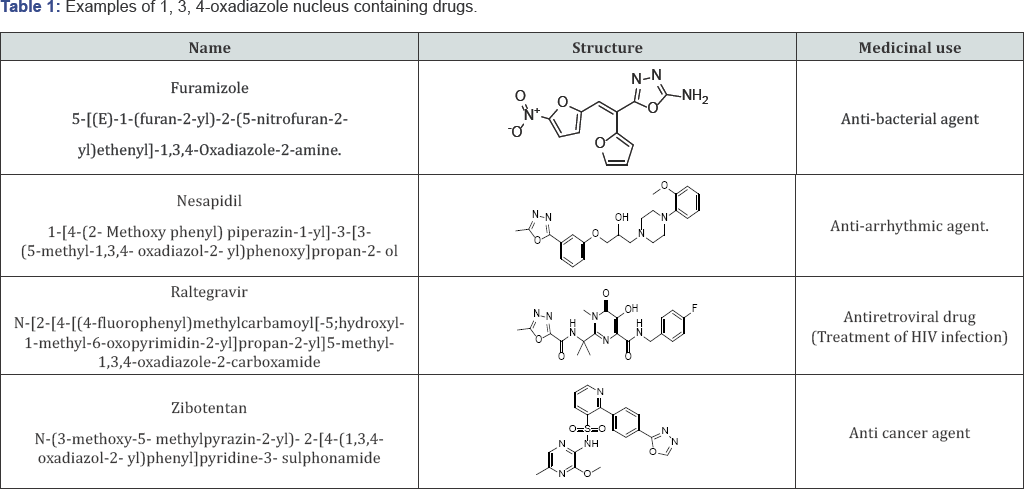
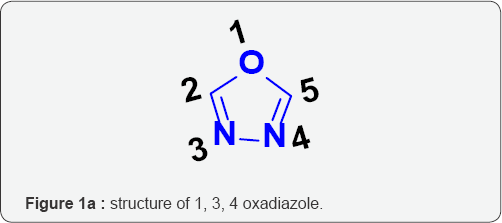
The development of new antimicrobial and cytotoxic agents is one of the fundamental goals in medicinal chemistry. In recent years, there has been a concerned search for the discovery and development of potent and selective cytotoxic and antimicrobial agents. N-heterocyclic ring systems are important for the drug design, among these Pyrimidine and 1,3,4-oxadiazole compounds are present in several classes of natural and synthetic biologically active compounds [1-7]. Oxadiazole (1) is five member cyclic compounds with one oxygen and two nitrogen atoms in the ring (Figure 1a-1d). 1, 3, 4-oxadiazoles have occupied unique place in the field of Medicinal chemistry due to their wide range of activities (2). The review of literature shows that 1, 3, 4-oxadiazole nucleus possess antimicrobial (3), antifungal (4), anti-inflammatory (5), anticonvulsant (6), antioxidant, Antihypertensive Activity (7-15), analgesic (7) and mutagenic activity (8).

Number of drugs available in the market such as tiodazosin, nosapidil, furamizole are 1, 3, 4-oxadiazole derivatives (9). Apartfrom these biological activities, 1,3,4-oxadiazole derivatives were found to have some material applications in the field of liquid crystals and photosensitizer (10). Literature survey reveals that1,3,4-oxadiazole derivatives posses a broad spectrum of biological activities (11-14). Antihypertensive Activity Consequently, they have attracted increasing attention in the field of drug discovery [8-10]. Attributable to such biological importance, Pyrimidine core structure derivatives have grown to be the synthetic goals of many organic and medicinal chemistry researchers.
Synthesis
Synthesis of 2-methyl-4-phenyl Pyrimidine (3)
A mixture of 3-phenylpropiolaldehyde (0.01 mol) and acetimidine hydro chloride (0.01 mol) was stirred in dry aceto nitrile (10 ml) and dry Na2CO3 (0.02mol) was added to it. The stirring was continued for 0.5 hr under Micro Wave conditions at 90Oc .Reaction progress was monitored by TLC [11,12]. After completion of reaction cool to RT. Then concentrated under reduced pressure by using Rota evaporator & Purified by column chromatography(100-200 mesh size silica) with elution of 10% Ethyl acetate to get pure yellow solid yield:47 % mp: 130Oc- 132Oc.
a. IR(KBr,cm-1): 3100 (Ar.C-H str.), 1628 (Ar.C-C str.), 1450(C-N str.);
b. !H NMR (DMS0-d6, ppm): 2.5(3H, S), 8.45(1H, d, J=7.4HZ), 7.8(1H, d, j=7.4HZ), 7.4-7.8(5H, m);
c. EI-Mass: 171 (M+, +Ve mode)
Synthesis of 4-phenylpyrimidine-2-carboxylic acid (4)
A mixture of compound (3) (0.01 mol), selenium Di oxide (0.05 mol), and pyridine (10 ml) was refluxed for 2 hours. Reaction progress was monitored by TLC. After completion of compound 3, concentrated under reduced pressure, then added water (10 ml), acidified with Conc. HCl, white solid was formed, filter off, dried, to get 75 % yield. M.P: 187-189OC.
a. IR (KBr,cm-1): 3100 (Ar.C-H str.), 1628 (Ar.C-C str.), 1450 (C-N str.),1720(C=0 str.),3200(-0H Str.),1310(C-0 str.);
b. !H NMR (DMS0-d6, ppm):9.35(1H,d, j=8.3 HZ),8.55(1H,d,j=8.3HZ),7.4-7.8(5H,m),10.5(-C00H);
c. EI-Mass: 199 (M+, -Ve mode)
Synthesis of methyl 4-phenylpyrimidine-2-carboxylate (5)
A mixture of compound (4) (0.05 mol, 10g), Sulphuric acid (catalytic), and Methanol (10 V, 100mL) was refluxed for 2 hours. Reaction progress was monitored by TLC. After completion of reaction concentrated under reduced pressure, then added Na2C03 Solution (10 ml), white solid was formed, filter off, dried, to get 75 % yield.
a. IR (KBr, cm-1): 3115(Ar.C-H str.), 1628 (Ar.C-C str.), 1450 (C-N str.), 1760(C=0 str.), 1755(-Carbonyl Str.), 1310(C-0 str.).
b. JH NMR (DMS0-d6, ppm):9.45(1H, d, j=8.3 HZ), 8.65(1H,d. j=8.3HZ),7.4-7.8(5H,m),3.85(-0-CH3);
c. EI-Mass: 215 (M+, +Ve mode)
Synthesis of methyl 4-phenylpyrimidine-2- carbohydrazide (5)
To a solution of compound (5) (0.05 mol, 10.8g),in ethyl alcohol (50 mL) was added with excess of 99% hydrazine hydrate and refluxed for 24 hr. Reaction mixture was concentrated and allowed to cool, separated crystals was filtered, dried and recrystallized from minimum amount of ethyl alcohol [13-15]. The obtained yield is 75%.
a) IR (KBr, cm-1): 3320 (-N-H str.)3110 (Ar.C-H str.), 1628 (Ar.C-C str.), 1450 (C-N str.), 1690(C=0 str.).
b) 1H NMR (DMS0-d6, ppm):9.45(1H, d, j=8.3 HZ), 8.65(1H, d,j=8.3HZ),7.4-7.8(5H,m),3.85(-0-CH3);
c) EI-Mass: 215 (M+, +Ve mode)
d) Synthesis of 2-phenyl-5-(4-phenylpyrimidin-2-yl)-1, 3, 4-oxadiazole (8a),
e) 2-(4-phenylpyrimidin-2-yl)-5-p-tolyl-1, 3, 4-oxadiazole (8b),
f) 2-(4-methoxyphenyl)-5-(4-phenylpyrimidin-2-yl)-1, 3, 4-oxadiazole (8c),
g) 2-(4-phenylpyrimidin-2-yl)-5-(4-(trifluoromethoxy) phenyl)-1, 3, 4-oxadiazole (8d),
h) 2-(4-phenylpyrimidin-2-yl)-5-(pyrazin-2-yl)-1, 3, 4-oxadiazole (8e),
i) 2-(4-nitrophenyl)-5-(4-phenylpyrimidin-2-yl)-1, 3, 4-oxadiazole (8f),
j) 2-(4-phenylpyrimidin-2-yl)-5-(pyridin-4-yl)-1, 3, 4-oxadiazole (8g),
k) 2-(4-phenylpyrimidin-2-yl)-5-(4-(trifluoro methyl) phenyl)-1, 3, 4-oxadiazole (8h),
l) 2-(2, 5-difluorophenyl)-5-(4-phenylpyrimidin-2-yl)-1,3, 4-oxadiazole (8i),
m) 2-(4-phenylpyrimidin-2-yl)-5-(thiophen-2-yl)-1, 3, 4-oxadiazole (8j)
To the equi molar mixture of compounds (6) and 7(a-j), catalytic amount of POCl3 was added and it was refluxed for 4-6 hrs. The reaction mixture was poured over crushed ice and neutralized by sodium carbonate solution. The precipitate formed was filtered and dried. The crude product was purified by the alcohol and DMF mixture.
a) IR (KBr, cm-1): 1320 (-C-F str.)3110 (Ar.C-H str.), 1628 (Ar.C-C str.), 1450 (C-N str.), 1354 (C-0-C).
b) 1H NMR (DMS0-d6, ppm):8.65(1H, d, j=7.3 HZ), 7.95(1H,
d, j=7.3HZ),7.4-7.8(5H,m),7.85(2H, d, j=7.6HZ); 8.15(2H, d, j = 7.6HZ);
c) 13C NMR (100 MHz; CDCl3): SC 125.5, 128.89, 130.55,133.45.141.149, 158.8, 162.34, 165.65.
d) EI-Mass: 369 (M+, +Ve mode)
e) IR (KBr, cm-1): 1340 (-C-F str.), 3108 (Ar.C-H str.), 1620 (Ar.C-C str.), 1458 (C-N str.), 1350 (C-0-C).
f) jH NMR (DMS0-d6, ppm):8.65(1H, d, j=7.3 HZ), 7.95(1H,
d, j=7.3HZ), 7.4-7.8(5H, m),7.55(1H, d, j=3.6HZ); 7.15(2H, d, j = 7.6HZ); 7.35 (2H, d, j = 7.6HZ).
g) 13C NMR (100 MHz; CDCl3): SC 125.5, 128.89, 130.55,133.45.141.149, 155.45, 158.8, 162.34, 165.45.
h) EI-Mass: 335 (M+, -Ve mode)
i) IR (KBr, cm-1): 740 (-C-S-C str.), 3108 (Ar.C-H str.), 1620 (Ar.C-C str.), 1455 (C-N str.), 1350 (C-0-C).
j) !H NMR (DMS0-d6, ppm):8.55(1H, d, j=7.3 HZ), 7.93(1H,
d, j = 7.3HZ), 7.4-7.8(5H,m),7.65(1H, d, j = 7.6HZ); 7.15(1H, d, j = 7.6HZ); 7.75 (1H, d, j = 7.6HZ).
k) 13C NMR (100 MHz; CDCl3): SC 125.5, 128.89, 130.55,133.45.141.149, 155.45, 158.8, 162.34, 165.45.
l) EI-Mass: 307 (M+, +Ve mode)
Biological Activity
Antibacterial studies
The newly prepared compounds were screened for their antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumonia and Escherichia coli (clinical isolate) bacterial strains by disc diffusion method (15, 16). A standard inoculums (1-2x107 c.f.u./ml 0.5 McFarland standards) were introduced on to the surface of sterile agar plates, and a sterile glass spreader was used for even distribution of the inoculums. The disks measuring 6 mm in diameters were prepared from what man no. 1 filter paper and sterilized by dry heat at 140°C for 1 h. The sterile disks previously soaked in a known concentration of the test compounds were placed in nutrient agar medium. Solvent and growth controls were kept. Amoxicillin (30 ^g) was used as positive control and the disk poured in DMSO was used as negative control and the test compounds were dissolved in DMSO at concentration of 100 and 50 ^g/ml. The plates were inverted and incubated for 24 h at 37°C. The susceptibility was assessed on the basis of diameter of zone of inhibition against Gram-positive and Gram-negative strains of bacteria. Inhibition of zone of measured and compared with controls. The bacterial zone of inhibition values are given in (Table 1). The order of activity was 8j>8i>8h>8g>8d>8f>8b>8c>8e>8a.
Antifungal studies
The newly prepared compounds were screened for their antifungal activity against Candida albicans and Aspergillus flavus in DMSO by agar diffusion method (17). Sabourauds agar media was prepared by dissolving peptone (1 g), D-glucose (4 g) and agar (2 g) in distilled water (100 ml) and adjusting pH 5.7. Normal saline was used to make suspension of corresponding species. Twenty milli liters of agar media was poured into each Petri dish. Excess of suspension was decanted and the plates were dried by placing in an incubator at 37°C for 1 h using an agar punch, wells were made and each well was labeled [16,17]. A control was also prepared in triplicate and maintained at 37°C for 3-4 days. The fungal activity of each compound was compared with Flucanazole as a standard drug. Inhibition zone were measured and compared with the controls. The fungal zone of inhibition values are given in (Tables 2 & 3).
Acknowledgements
The authors express their sincere thanks to the Managing Director, Dr. Maddula Sreenivas Reddy, Prajna Generics Private Limited, Hyderabad for providing the spectral data. We are also thankful to the Director, Prof V V Basava Rao for Encouragement,Department of Bio Technology, Sri Krishnadevaraya University,and Ananthapuramu for the Biological studies.
References
- Navin B Patel, Jaymin C Patel (2010) Synthesis and Antimicrobial Activity of 3-(1,3,4-Oxadiazol-2-yl) quinazolin-4(3H)-ones. Scientia Pharmaceuica 78(2): 171-193.
- Jnyanaranjan Panda, V Jagannath Patro, Chandra Sekhar Panda, Jitendriya Mishra (2011) Synthesis, Characterization, Antibacterial and Analgesic Evaluation of some 1,3,4-Oxadiazole Derivatives. Der Pharma Chemica 3(2): 485-490.
- Rakesh R Somani, Anuj G Agrawal, Pushkar P Kalantri, Pratibha S Gavarkar, Erik De Clerq (2011) Investigation of 1,3,4-OxadiazoleScaffold as Potentially Active Compounds. International Journal of Drug Design and Discovery 2(1): 353-360.
- Ponnilavarasan Ilangovan, Ayaluraja Sekaran, Sundaramoorthi, Chenniappan, Bhalchandra Keshao Chaple (2011) Synthesis, Characterization and Antimicrobial Activity of 1, 3, 4-Oxadiazole Derivatives. Journal of Pharmacy Research, Tamilnadu India 4(6): 1696-1698.
- Rakesh Saini, Saurabh Chaturvedi, Achyut Narayan Kesari, Swatrantra Kushwaha (2010) Synthesis of 2-(Substituted)-5-(Benzotriazomethyl)-1,3,4- Oxadiazole for Anti-fungal Activity. Der Pharma Chemica, Kanpur, India, 2(2): 297-302.
- Mohd Amir, SA Javed, Harish Kumar (2007) Synthesis, Characterization and Biological Evaluation of Pyrazolones Containing Multi Substituted Thiazolidinones and Oxadizoles. Indian Journal of Chemistry pp. 10141019.
- Cruickshank R, Duguid JP, Marmion BP, Swain RHA (1975) Medicinal microbiology. Churchill Livingstone, London, UK.
- Poonam Singh, Pankaj K, Jangra (2010) Der Chemica Sinica 1(3): 118123.
- M Vijey Aanandhi, Mohammed Hashim Mansoori, S Shanmugapriya, Shiny George, P Shanmugasundaram (2009) Research Journal of Pharmaceutical, Biological and Chemical Sciences. pp. 223-233.
- Vijay V Dabholkar, Nitin V Bhusari (2011) International Journal of Chemical, Environmental and Pharmaceutical Research. 2(1): 1-4.
- Nagaraj, Chaluvaraju KC, Niranjan MS, Kiran S (2011) International Journal of Pharmacy and Pharmaceutical Sciences 3(3): 9-16.
- Mogilaiah, GR Sudhakar (2001) Indian J Het Chem 11: 163.
- Linhong Jin, Jiang Chen, Baoan Song, Zhuo Chen, Song Yang, et al. (2006) Bioorganic & Medicinal Chemistry Letters. pp. 5036-5040.
- Harish Kumar, Sadique A Javed, Suroor A Khan, Mohammad Amir (2008) European Journal of Medicinal Chemistry 43: 2688-2698.
- Asif Husain, Mohammed Ajmal (2009) Acta Pharm 59: 223-233.
- Collins AH (1976) Microbiological methods Butterworth, London, UK.
- Varma RS (1998) Anti-fungal agents: past, present and future prospects. National Academy of Chemistry & Biology, Lucknow, India.






























