Thermal Alkylation of Trimethylphenacyl Ammonium Bromide with Benzyldimethylphenyl Ammonium Chloride
GH Torosyan*
National polytechnic university of Armenia, Asia
Submission: October 02, 2017; Published: October 17, 2017
*Corresponding author: GH Torosyan, Doctor of Chemical sciences professor, National polytechnic university of Armenia, Asia, Email: gagiktorosyan@seua.arn
How to cite this article: G Torosyan. Thermal Alkylation of Trimethylphenacyl Ammonium Bromide with Benzyldimethylphenyl Ammonium Chloride. Organic & Medicinal Chem IJ. 2017; 4(2): 555631. DOI: 10.19080/OMCIJ.2017.04.555631.
Short Communication
The alkylating ability of quaternary ammonium salts (Quat) has been known for a long time [1-3]. Alkylation with Quat-s is advantageous both in cases where the alkyl ammonium salt is more accessible than the alkyl halide and in those cases where the alkylated compound is unstable under alkylation conditions by alkyl halides (for example, in alkaline media). Considering this circumstance in this report turned to the almost forgotten method of alkylation of the element-H acids by Quat-s.
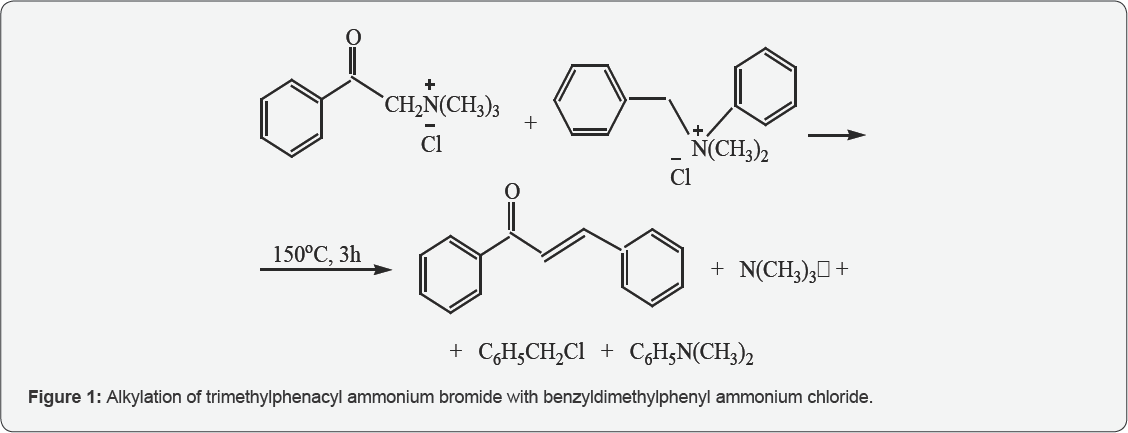
It was previously found that the series of Quat-s reacts with alkyl halides as a C-H acid, on the basis of which the alkylation- elimination reaction in Quat was discovered. However, there is no literature on alkylation of Quat with the alkyl ammonium salt itself. At one time, in order to confirm the intermolecular nature of the discovered reaction, Stevens subjected a rearrangement a mixture of two Quat, each of which was able to rearrange separately. The absence of a mixed rearrangement product confirmed the intermolecular nature of the reaction. However, confirming the intermolecular nature of the rearrangement Stevens did not rule out the possibility of intermolecular C-alkylation in those cases where the intermolecular mechanism, if not excluded is at any rate difficult. The possibility of thermal alkylation with alkyl ammonium salts has been investigated. The alkylating agent was benzyldimethylphenyl-ammonium chloride (Figure 1).
As a result, it has been established that intermolecular alkylation with an alkyl ammonium salt proceeds. As in the case of the reaction alkylation-eliminitation, there is also a ^-elimination take place with the formation of a chalcone. It should be noted that the yield of the chalcone is higher here than in the case of the alkylation-elimination reaction in an aqueous alkaline medium. In order to prevent the products of the reaction from becoming smeared, the process is carried out in the presence of an antioxidant (neozone D). Thus, a thermal alkylation-elimination reaction has been detected in a series of Quaternary ammonium salts [4,5].
Experimental Part
Alkylation of trimethylphenacyl ammonium bromide with benzyldimethylphenyl ammonium chloride
A mixture of equimolar amounts of the starting ammonium salts was heated in 140oC at 3 hours. The reaction mixture was then diluted with water and extracted with diethyl ether. The latter was washed with a titrated hydrochloric acid solution and dried on MgSO4. The non-amino products of the reaction were isolated after diethyl ether distillation. The aqueous layer was treated with potash. Volatile amines were absorbed by the system of absorbers with a titrated solution of hydrochloric acid. Reverse titration determined the amount of volatile amine. High-boiling amines were isolated from the aqueous layer by extraction with diethyl ether. By evaporation of the aqueous layer to dryness and extraction with absolute alcohol, unselected portions of the initial Quat were isolated [6].
From 1,03gr (0,004 mol) of trimethylphena-cyl -am-monium bromide (Mp. 191-192oC) and 0,99gr (0,004mol) of benzyldimethylphenyl ammonium chloride (Mp. 135-137oC) was heated in 150oC at 3 hours, It had been obtained 0,25 gr chalcone - 30,0 0%, 0, 18gr dimer of chalcone - 21,4 0% , 0,073gr benzyl chloride - 14,4%, 0,32gr dimethyl aniline- 66,1%Returned 0, 68 gr mixture of initial QUAT.
Conclusion
The structure synthesized chalcone was proved by IR, UV,1HNMR and Mass spectrometry
References
- E Wedekind, E Pascheke (1910) Influence of cogitations of the velocity of decomposition of Quaternary ammonium salts. Ber J 43: 1303.
- HR Snyder, CW Smith, JMC Stewart (1944) Alkylation with quaternary ammonium salts. JA Chem 66 (2): 200.
- GH Torosyan (1987) Reaction of inter and internal alkylation for compounds with carbonyl and hydroxyl groups. Thesis for chemical doctor degree, Yerevan, Armenia pp.358.
- GH Torosyan (2017) Alkylation-Elimination Reaction in Quaternary Ammonium Salts. Organic & Medicinal Chem IJ 3(3): 555613.
- TS Stevens, WW Snedelen, ER Stiller, T Tompson (1930) Degradation of quaternary ammonium. J Chem Soc 2119.
- JA Vanecko, H Wan, FG West (2006) Recent advances in the Stevens rearrangement of ammonium ylides. Application of the synthesis of alkaloid natural products. Tetrahedron 62(2006): 1043-1062.






























