Multi Component Green Synthesis of Bis-Pyrrol Indoline-2-Ones Triazoles Catalysed By Cu NPS on Activated Carbon in Water
B Saritha*, P Venkata Ramana, D Madhavi and LK Ravindranath
Department of Chemistry, Sri Krishnadevaraya University, India
Submission: September 18, 2017; Published: October 03, 2017
*Corresponding author: B Saritha, Department of Chemistry, Sri Krishnadevaraya University, Anantapur 515 003 (AP), India; Email: saritha246chinni@gmail.com
How to cite this article: B Saritha, P Venkata, D Madhavi, LK Ravindranath. Multi Component Green Synthesis of Bis-Pyrrol Indoline-2-Ones Triazoles Catalysed By Cu NPS on Activated Carbon in Water. Organic & Medicinal Chem IJ. 2017; 4(1): 555630. DOI: 10.19080/OMCIJ.2017.04.555630.
Abstract
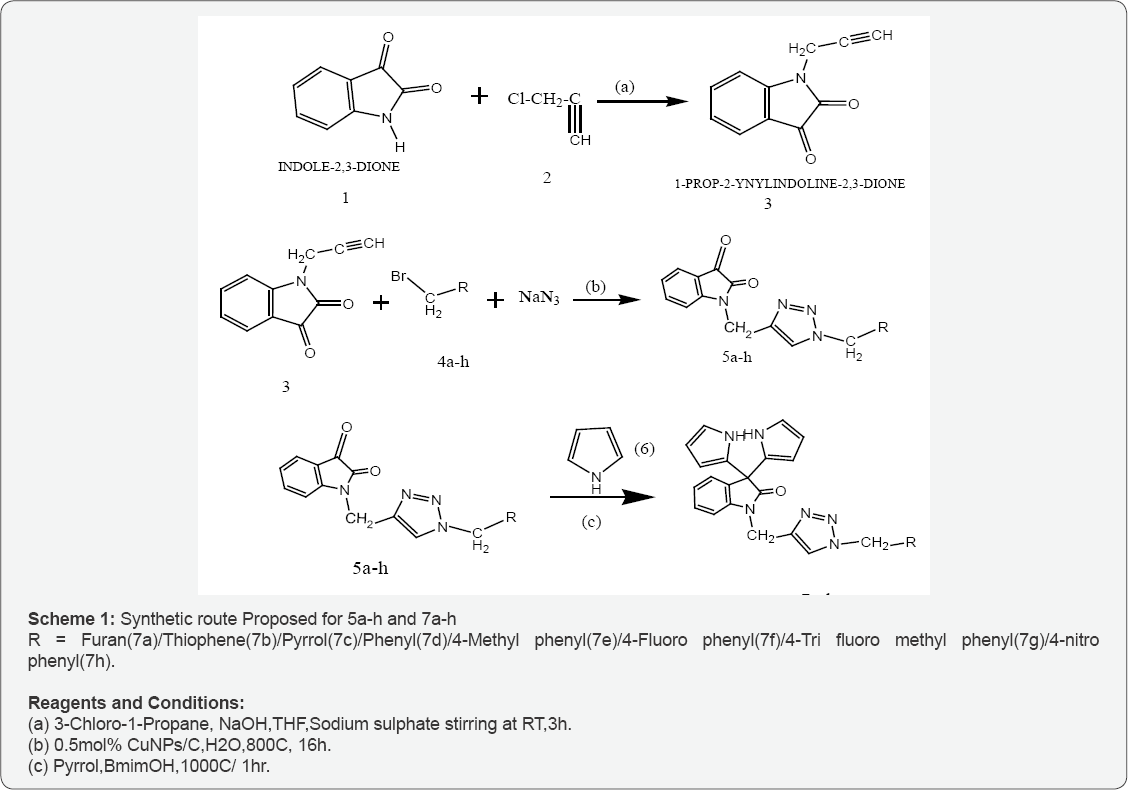
A variety of potentially biological active multi component green synthesis of Bis-pyrrol indoline-2-ones triazoles possessing N- alkyl furan/ N- alkyl thiophene /N- alkyl pyrrol by catalysed Cu-NPs on activated carbon in water involving green synthetic technology have been developed. It involves in three steps. The first step involves the synthesis of 1-prop-2-ynyl indoline-2,3-dione(3) from indoline -2,3-dione and 3-chloropropanone(2). The intermediate1-prop-2-ynyl indoline-2,3-dione(3) was converted into 1-((1-furan/thiophene/pyrrol/phenyl/4- methyl phenyl/4-fluoro phenyl/4- trifluro methyl phenyl/4-nitro phenyl)-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)indiline-2,3-dione (5a-h) by 1,3- dipolar cyclo addition with 2-bromo methyl-1H-furan/ 2-bromo methyl-1H-thiophene/ 2-bromo methyl-1H-pyrrol benzyl bromide/4-methyl benzyl bromide/4-fluoro benzyl bromide/4-tri fluoro benzyl bromide/4- nitro benzyl bromide (4a-h) catalysed by Cu-NPs on activated carbon catalysed click reaction in neat water depicted in step-2. The step three involves green synthesis of 1-((1-furan/thiophene/pyrrol/phenyl/4- methyl phenyl/4-fluoro phenyl/4- trifluro methyl phenyl/4-nitro phenyl)-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl) -3,3-di(1H-pyrrol-2-yl) indoline-2-ones(7a-h) from 1-((1-1-((1-furan/ thiophene/pyrrol/phenyl/4-methyl phenyl/4-fluoro phenyl/4- trifluro methyl phenyl/4-nitro phenyl)-2-yl)-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)indiline-2,3-dione(5a-h) and pyrrol (6) in presence of selected ionic liquid. The newly synthesized triazoles (7a-h) were characterized by spectral analysis. The synthetic route was shown in scheme-1. Antimicrobial activity of 7a-h was studied in presence of Ag NPs by disk diffusion method.
Keywords: Click Reaction; (Bmim) Oh (1-Butyl-3-Methylimidazolinium Hydroxide) Bis-Pyrrol Indoline Triazoles; Cu-Nps; Eco-Friendly Synthesis; Ag-Nps.
Introduction
Heterocycles play an important role in biochemical processes and, therefore, they are very frequently found as substructures in numerous pharmaceutical products [1]. Among them, 1,2,3- and 1,2,4-triazoles are known to possess remarkable biological properties as antitumor, antiviral, anti-inflamMicrobial Experimentationory, analgesic, antifungal or antibacterial agents [2]. Nowadays, click chemistry[3] represents a pivotal tool in the discovery of new therapeutic compounds and in medicinal chemistry, since it allows molecular diversity in a direct, precise and selective manner.[4] In particular, the Huisgen 1,3-dipolar cycloaddition of azides and alkynes [5] have become a synthetic cornerstone, the paramount discovery by the groups of Meldal [6] and Sharpless [7] of its copper(I)-catalysed version (CuAAC). [8] This powerful methodology, which is considered the paradigm of a click reaction, gives a straight access into the 1,2,3-triazole moiety with high reliability and selectivity. This particular nucleus is used as a robust linker of complexes On the other hand, owing to our dedication to study and understand the reactivity of active metals and nanoparticles, [9] molecular architectures of significance in different fields, and especially in synthetic routes to bioactive molecules [10]. Copper nanoparticles (CuNPs) were formed either from CuCl22H2O or anhydrous CuCl2 under the aforementioned conditions. These CuNPs (10 mol%) effectively catalysed the 1,3-dipolar cycloaddition of organic azides and terminal alkynes in remarkably short reaction times (10-120 min) [11], the CuNPs underwent dissolution under the reaction conditions (Et3N, THF, 65 °C) the same could not be reused. In order to overcome this inconvenience, supported catalysts based on CuNPs were developed for better recyclability and stability than the unsupported counterparts. [12] In this sense, a catalyst consisting of oxidized copper nanoparticles on activated carbon, readily prepared under mild conditions, which manifested a high versatility in the multicomponent Huisgen 1,3-dipolar cycloaddition in water. [13] The organic halides, azides as aryldiazonium salts, anilines, epoxides and alkene, were proven to be appropriate substrates in this process. In order to expand the applicability of this methodology, we have reported here in our results on the multicomponent synthesis of an array of 1,2,3-triazoles, derived from some natural products and a synthetic one, with potential biological activity.
Bis Pyrrol-2-yl-indoline-2 ones derivatives have been drawing the attention of synthetic organic chemists due to their wide spectrum of biological properties [14]. 3,3-Diaryloxindoles known to exhibit a wide range of biological activities such as antibacterial [15], antiprotozoal [16], anti-inflamMicrobial Experimentationory [17] and anticancer activity [18]. A reaction which involves ionic liquids as catalysts [19] and /or media [20] in reactions have been widely used in organic Trans forMicrobial Experimentationions due to their advantages such as good solvating ability, negligible vapor pressure, high polarity and ease of work-up. [Bmim]OH (1-butyl-3-methylimidazolinium hydroxide) is one such task-specified ionic liquid which acts as reaction medium as well as a basic catalyst and has got varied applications [21] in the field of synthetic methodology development. In view of our continued interest in indoles [22] and the development of green procedures for the synthesis of diverse heterocyclic compounds of biological significance, we now report a simple and efficient method for the synthesis and use of Cu NPs of Bis-Pyrrol indoline triazoles 7a-h using [Bmim] OH as a task-specific ionic liquid.
Experimental Section
Melting points were obtained with a Reichert Thermovar apparatus and are un corrected. Infrared analysis was performed with a Jasco 4100LE (Pike MIRacle ATR) spectrophotometer; wavenumbers are given in cm-1. NMR spectra were recorded on Bruker Avance 400 spectrometers (400 MHz for 1H NMR; 100 MHz for 13C NMR); chemical shifts are given in (5) parts per million and coupling constants (J) in Hertz. Activated charcoal (Norit CA1, Aldrich), and sodium azide (Across) were commercially available. All the starting Microbial Experimentationerials and other reagents were commercially available of the best grade (Aldrich) and were used without further purification. THF was dried in a Sharlab PS-400-3MD solvent purification system using an alumina column. Propargylation of the substrates was done following a literature procedure [23].
General Procedure for the synthesis of (3):
To a stirred suspension of sodium hydroxide (1.0 g, 42 m mol, and 1:2 eq.) in THF (25 ml) at room temperature, Indole-2,3- dione(1) was added at room temperature and the reaction mixture was stirred at same temperature for 30 min, 3-chloro -1-propyne (7.01 g, 42.0 mmol, 1:2 eq) was added drop wise to the stirring solution and the reaction mixture was stirred for an additional 3 hr. The progress of the reaction was monitored by TLC with hexane and ethyl acetate (7:3) as mobile phase, after completion of the reaction, the reaction mixture was poured on to cooled water, then extracted with ethyl acetate twice (2x20M). The combined organic layer was washed with water, brine, dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure to get crude compound of (3), then the crude compound was purified by silica gel column chroMicrobial Experimentationography, eluted with 5% EtOAc and 95% pet ether to afford pure 1-prop-2-ynyl indoline-2,3-dione(3). The yield of the compound was 70% with melting point 139-1410C. The structure of compound was characterized by spectral data (IR and 1H-NMR,) and elemental analysis.
Spectral data of Compound (3):
The IR(KBr) spectra of 1-Prop-2-ynyl indoline-2,3-dione(3) was recorded in the range of 4000-400 cm'1 in KBr pellet reflect the molecular structure and showed the characteristics bands 3250 cm-1 (C =C-H str.),2861 and 2841 cm-1(Aliphatic CH str.),2150 cm-1 (C = C str.),1743 cm-1 and 1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline2.3- dione). 1H-NMR (400MHz, DMSO-d6) spectrum of 1-Prop- 2-ynyl indoline-2,3-dione(3) was recorded in DMSO-d6 showed the signals at 8 PPm 7.19-7.83 (m,4H,Ar-H protons), 3.70 (s, 2H,CH2 attached to (nitrogen of indoline-2,3-dione) and 2.25(s, H, acetylinic-CH proton) Anal. Caled.(%) for C11H7NO2: C:71.35,H:3.78,N:7.56, found C: 69.25,H:2.95,N:6.75.
General procedure for the synthesis of (5a-h):
A mixture of NaN3 (72 mg. 1.1mmol) 2- bromo methyl furan(4a)/ 2-bromo methyl thiophene(4b)/ 2-bromo methyl pyrrol (4c) (1m mol) benzyl bromide/4-methyl benzyl bromide/4-fluoro benzyl bromide/4-tri fluoro benzyl bromide/4- nitro benzyl bromide and1- prop-2-ynyl indoline-2,3-dione(3) (1 m mol) were added to a suspension of Cu NPs/C (20mg. 0.5 mol% Cu) in H2O (2mL) . The reaction mixture was warmed to 700C and monitored by TLC and /or GLC until total conversion of the starting Microbial Experimentationerials. Water (30 mL) was added to the resulting mixture, followed by extraction with EtoAc (3x10 mL). The collected organic phases were dried with anhydrousMgSO4 and the solvent was removed in vacuum to give the corresponding (5a-h) which were purified by recrystellisation in EtoAc.
Spectral data for the Compound-5a:
1-((1-furan-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione(5a),Yield 70%, M.P-154-1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743-1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2.3- dione), 1500,1250,1000,850 cm-1 (Characteristics of furan signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-furan-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5a) was recorded in DMSO-d6 showed the signals at 8 PPm 6.18-7.83(m,7H,C6H4and C4H3 rings),2.35(s,2H,CH2-flankedbetween indolone nucleus and 1.2.3- triazole system), 4.84 (s, 2H, CH2-flanked between1,2,3- triazole system and furan ring.7.17(s,H,1,2,3-triazole ring),7.21(unequal doublet, H,CH attached to oxygen atom of furan ring). Anal. Caled.(%) for C18H19N4O3: C:63.52,H:5.92,N:16.46, found C:61.31,H:4.82,N:14.23.
Spectral data for the Compound-5b:
1-((1-thiophene-2-yl)methyl)-1H-1,2,3-triazole-4- yl) methyl) indoline-2,3-dione(5b), Yield70% M.P-154- 1560C,IR-(KBr) 2861 and 2841cm'1(Aliphatic CH str.),1743- 1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm'1 (C-N str, of indoline 2,3-dione), 3100,1400,1100,850,700 cm-1 (Characteristics of thiophene signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-thiophene-2-yl] methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5b) was recorded in DMSO-d6 showed the signals at 8 PPm 6.18-7.83(m,7H,C6H4and C4H3 rings),2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.84 (s, 2H, CH2-flanked between1,2,3-triazole system and thiophene ring), 7.17(s,H,1,2,3-triazole ring),6.91(unequal doublet ,H,CH attached to sulphur atom of thiophene ring). Anal. Caled.(%) for C18H19N4O2 S: C:60.65,H:5.66,N:15.72, found C:58.34,H:3.33,N:13.41.
Spectral data for the Compound-5c:
1-((1-pyrrol-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione(5c), Yield70% M.P-154-1560C,IR-(KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2.3- dione), 1600, 1400, 3500, 720 cm-1 (Characteristics of pyrrol signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-pyrrol-2-yl) methyl)-1H-1,2,3-triazole-4' yl) methyl) indoline-2,3-dione, (5c) was recorded in DMSO-d6 showed the signals at 8 PPm 6.18-7.83(m,7H,C6H4and C4H3 rings),2.35(s,2H,CH2-flanked between indolone nucleus and 1.2.3- triazole system), 5.03 (s, 2H, CH2-flanked between1,2,3- triazole system and pyrrol ring), 7.17(s,H,1,2,3-triazole ring),6.91(unequal doublet ,H,CH attached to NH atom of pyrrol ring). Anal. Caled.(%) for C18H20N5O2: C:63.89,H:5.96,N:20.76, found C:58.44,H:3.53,N:18.43
Spectral data for the Compound-5d:
1-((1-phenyl-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione(5d), Yield 70%,M.P-154-1560C,IR-(KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2.3- dione), 3000,2000-1600,1500,1000 cm-1 (Characteristics of phenyl signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-phenyl-2-yl) methyl)-1H-1,2,3-triazole-4' yl) methyl) indoline-2,3-dione, (5d) was recorded in DMSO-d6 showed the signals at 8 PPm 6.18-7.83(m,7H,C6H4and C4H3 rings),2.35(s,2H,CH2-flanked between indolone nucleus and 1.2.3- triazole system), 5.03 (s, 2H, CH2-flanked between1,2,3- triazole system and phenyl ring), 7.17(s,H,1,2,3-triazole ring),6.36(unequal doublet ,H,CH attached to NH atom of phenyl ring). Anal. Caled.(%) for C20H20N4O2:C:68.95,H:5.79,N:16.08,found C:57.43,H:4.56,N:14.06.
Spectral data for the Compound-5e:
1-((1-(4-methylphenyl-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione(5e), Yield70% M.P-154-1560C,IR- (KBr) 2861 and 2841cm-1 (Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2.3- dione), 3200, 2950, 1600-1500, 1100 cm-1(Characteristics of 4-methyl phenyl signals), 3030, 1500, 1100, 810, 720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-4-methyl phenyl -2-yl) methyl)- 1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5e) was recorded in DMSO-d6 showed the signals at 8 PPm 7.0-7.5(m,9H,C6H4and C6H5 rings),2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3-triazole system and benzene ring), 7.30(s,H,1,2,3-triazole ring), Anal. Caled.(%) for C21H22NO2: C:69.59,H:6.12,N:15.46, found C:66.48,H:5.10,N:14.23.
Spectral data for the Compound-5f:
1-((1-(4-fluorophenyl-2-yl)methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione (5f),Yield70% M.P-154-1560C,IR- (KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2.3- dione), 3000,1600,1500, 1210, 900,750cm-1(Characteristics of 4-fluorophenyl signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-4-fluorophenyl -2-yl) methyl)- 1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5f) was recorded in DMSO-d6 showed the signals at 8 PPm 7.0- 7.5(m,9H,C6H4and C6H5 rings), 2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3-triazole system and benzene ring), 7.320(s,H,1,2,3-triazole ring), Anal. Caled.(%) for C20H22N4O2: C:65.56,H:5.2 3,N:15.29, found C:63.33,H:4.12,N:13.15.
Spectral data for the Compound-5g:
1-((1-(4-trifluorophenyl-2-yl)methyl)-1H-1,2,3-triazole- 4-yl) methyl) indoline-2,3-dione (5g),Yield70% M.P-154- 1560C,IR-(KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743- 1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2,3-dione), 1350-1150,1000,1550,850,750 (Characteristics of 4-trifluorophenyl signals),3030, 1500, 1100, 810,720cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-4-tri fluorophenyl -2- yl) methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5g) was recorded in DMSO-d6 showed the signals at 8 PPm 7.0- 7.5(m,9H,C6H4and C6H5 rings), 2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3-triazole system and benzene ring), 7.30(s,H,1,2,3-triazole ring), Anal. Caled.(%) for C21H19N4O2: C:60.57,H:4.60,N:13.46, found C:58.46,H:2.30,N:11.23.
Spectral data for the Compound-5h:
1-((1-(4-nitrophenyl-2-yl)methyl)-1H-1,2,3-triazole-4-yl] methyl) indoline-2,3-dione (5h), Yield70% M.P-154-1560C,lR- (KBr) 2861 and 2841cm-1(Aliphatic CH str.),1743-1691cm-1 (C=O) of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2,3-dione), 3100, 2900, 1620, 1420,1000,850,800cm- 1 (Characteristics of 4-nirophenyl signals),3030, 1500, 1100, 810,720cm-1 (Characteris tics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-4-tri fluorophenyl -2- yl) methyl)-1H-1,2,3-triazole-4-yl) methyl) indoline-2,3-dione, (5g) was recorded in DMSO-d6 showed the signals at 8 PPm7.0- 7.5(m,9H,C6H4and C6H5 rings), 2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3-triazole system and benzene ring), 7.30(s,H,1,2,3-triazole ring), Anal. Caled.(%) for C20H19N5O4: C:61.06,H:4.87,N:17.80, found C:57.03,H:2.43,N:14.12.
General procedure for the synthesis of (7a-h):
A mixture of 1-((1- furan/thiophene/pyrrol/phenyl/4- methyl phenyl/4-fluoro phenyl/4- trifluro methyl phenyl/4-nitro phenyl)-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)indiline-2.3- dione/1-((1-thiophene-2-yl)methyl)-1H-1,2,3-triazol-4-yl] methyl)indoline-2,3-diones(5a-h) (1.0 mmol) and pyrrol (6, 2.00 mmol) and [Bmim]OH (10 ml) was heated at 1000 C until the completion of reaction as checked by TLC. To the resulting oily reaction mixture was added ethanol (10 ml) to force out the crude product from the polar ionic liquid reaction medium. The separated solid mass was collected by filtration and dried in oven to obtain crude (7a-h). The latter, were recrystallized from ethanol to get the pure (7a-h). The filtrate consisting of the ionic liquid and ethanol were evaporated to remove ethanol and the recovered ionic liquid was reused for subsequent reactions. To compensate for the loss of some ionic liquid during the work up procedure, an amount (5 ml) of fresh [Bmim] OH was added after the 4 runs of the reactions.
Spectral data for the Compound-7a:
1-((1-(1-furan-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl) indoline -2-one(7a), Yield 70%, M.P-154- 1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743- 1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2,3-dione), 1500,1250,1000,850 cm-1 (Characteristics of furan signals),3030,1500,1100,810,720 cm-1 (Characteristics of1.2.3- triazole system),3 500,1600,1400,72 0cm-1(Characteristics of pyrrol signals) 1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-(1-furan-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl) indoline -2-one(7a), was recorded in DMSO-d6 showed the signals at δ PPm 7.0-7.50(m,7H,C6H4and C4H3 rings),5.90-6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2- flankedbetween indolone nucleus and 1,2,3-triazole system), 4.84 (s, 2H, CH2-flanked between1,2,3-triazole system and furan ring.7.17(s,H,1,2,3-triazole ring),7.21(unequal doublet, H,CH attached to oxygen atom of furan ring). Anal. Caled.(%) for C11H7NO2: C:71.35,H:3.78,N:7.56, found C: 69.25,H:2.95,N:6.75.
Spectral data for the Compound-7b:
1-((1-(1-thiophene-2-yl)methyl)-1H-1,2,3-triazol-4-yl) methyl)-3,3-di(1H-pyrrol-2-yl) indoline -2-one(7b), Yield 70%, M.P-154-1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743-1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2,3-dione), 3100,1400,1100,850,700 cm-1 (Characteristics of thiophene signals), 3500,1600,1400,720cm- Characteristics of pyrrol signals)3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-(1-thiophene-2-yl)methyl)-1H-1.2.3- triazol-4-yl)methyl)-3,3-di(1H-pyrrol-2-yl) indoline -2-one (7b), was recorded in DMSO-d6 showed the signals at δ PPm 7.0- 7.50(m,7H,C6H4and C4H3 rings),5.90-6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2-flankedbetween indolone nucleus and 1.2.3- triazole system), 4.99 (s, 2H, CH2-flanked between1,2,3- triazole system and thiophene ring.7.17(s,H,1,2,3-triazole ring),6.91(unequal doublet, H,CH attached to sulphur atom of thiophene ring).
Spectral data for the Compound-7c:
1-((1-(1-pyrrol-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl) indoline -2-one(7c), Yield 70%, M.P-154- 1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743- 1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2,3-dione), 1600,1400,3500,720 cm-1 (Characteristics of pyrrol signals),3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-(1-pyrrol-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl) indoline -2-one (7c), was recorded in DMSO-d6 showed the signals at 8 PPm 7.0-7.50(m,7H,C6H4and C4H3 rings),5.90-6.95(m,9H,tri-pyrrol ring),2.35(s,2H,CH2- flankedbetween indolone nucleus and 1,2,3-triazole system), 5.03 (s, 2H, CH2-flanked between1,2,3-triazole system and pyrrol ring. 6.36(s,H,1,2,3-triazole ring),6.91(unequal doublet, H,CH attached to NH atom of pyrrol ring).
Spectral data for the Compound-7d:
1-((1--phenyl-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)-3.3- di(1H-pyrrol-2-yl)indoline -2-one(7d), Yield 70%, M.P-154- 1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743- 1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str, of indoline 2,3-dione), 3000,2000-1600,1500,1000 cm-1 (Characteristics of phenyl signals),3500,1600,1400,720cm-1 (Characteristics of pyrrol signals), 3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of 1-((1-(1-phenyl-2-yl)methyl)-1H-1,2,3- triazol-4-yl)methyl)-3,3-di(1H-pyrrol-2-yl) indoline -2-one (7d), was recorded in DMSO-d6 showed the signals at 8 PPm7.0- 7.50(m,9H,C6H4and C6H5 rings),5.90-6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2-flankedbetween indolone nucleus and1.2.3- triazole system), 2.35 (s, 2H, CH2-flanked between1,2,3- triazole system and phenyl ring. 7.42(s,H,1,2,3-triazole ring).Anal. Caled.(%] for C30H34N6O: C:72.85,H:6.93,N:16.99, found C:70.43,H:5.52,N:14.54.
Spectral data for the Compound-7e:
1-((1-(4-methylphenyl-2-yl)methyl)-1H-1,2,3-triazol-4-yl] methyl]-3,3-di(1H-pyrrol-2-yl] indoline-2-one(7e], Yield 70%, M.P-154-1560C,lR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.],1743-1691 cm-1(C=O of indoline 2,3-dione] and 1294 cm-1 (C-N str. of indoline 2,3-dione], 3200,2900,1600,1500,1100- 1000cm'1 (Characteristics of 4-methyl phenyl signals], 3500,1600,1400,720cm-1(Characteristics of pyrrol signals],3030,1500,1100,810,720 cm-1 (Characteristics of1.2.3- triazole system],1H-NMR (400MHz, DMSO-d6) spectrum of(( 1-(1-(4-methyl phenyl-2-yl)methyl)-1H-1,2,3-triazol' 4-yl]methyl]-3,3-di(1H-pyrrol-2-yl] indoline -2-one (7e], was recorded in DMSO-d6 showed the signals at δ PPm 7.0- 7.50(m,9H,C6H4and C6H5 rings],5.90-6.95(m,6H,bis-pyrrol ring],2.35(s,2H,CH2-flankedbetween indolone nucleus and1.2.3- triazole system], 2.35 (s, 2H, CH2-flanked between 1,2,3- triazole system and4-methyl phenyl ring]. 7.42(s,H,1,2,3-triazole ring]. Anal. Caled.(%] for C31H36N6O: C:73.20, H:7.13,N:16.52, found C:71.12,H:5.11,N:14.31.
Spectral data for the Compound-7f:
1-((1-(4-fluoro phenyl-2-yl)methyl)-1H-1,2,3-triazol-4-yl]methyl]-3,3-di(1H-pyrrol-2-yl] indoline-2-one(7f],Yield 70%, M.P-154-1560C,lR-(KBr] 2861 and 2841 cm- 1(Aliphatic CH str.],1743-1691 cm-1(C=O of indoline 2.3- dione] and 1294 cm-1 (C-N str. of indoline 2,3-dione], 3000,1600,1500,1210,900,750cm-1 (Characteristics of 4-fluoro phenyl signals], 3500, 1600, 1400, 720cm-1(Characteristics of pyrrol signals], 3030,1500,1100,810,720 cm-1 (Characteristics of 1,2,3-triazole system],1H-NMR (400MHz, DMSO-d6] spectrum of(( 1-(1-(4-fluoro phenyl-2-yl)methyl)-1H-1,2,3- triazol-4-yl]methyl]-3,3-di(1H-pyrrol-2-yl] indoline -2-one (7f], was recorded in DMSO-d6 showed the signals at PPm 7.0- 7.50(m,9H,C6H4and C6H5 rings],5.90-6.95(m,6H,bis-pyrrol ring],2.35(s,2H,CH2-flankedbetween indolone nucleus and 1.2.3- triazole system], 2.35 (s, 2H, CH2-flanked between1,2,3- triazole system and4-fluoro phenyl ring). 7.42(s,H,1,2,3-triazole ring]. Anal. Caled.(%] for C30H33N6O: C:70.29, H:6.49, N:16.39, found C:68.15,H:4.25,N:14.24.
Spectral data for the Compound-7g:
1-((1-(4-tri fluoro phenyl-2-yl)methyl)-1H-1,2,3- triazol-4-yl]methyl]-3,3-di(1H-pyrrol-2-yl] indoline-2-one(7g], Yield 70%, M.P-154-1560C,lR-(KBr] 2861 and 2841 cm-1(Aliphatic CH str.],1743-1691 cm-1(C=O of indoline2.3-dione] and 1294 cm-1 (C-N str. of indoline 2,3-dione], 1350-1150,1000,1550,850,750cm-1(Characteristics of 4-trifluoro phenyl signals), 3500, 1600, 1400,720cm-1 (Characteristics of pyrrol signals],3030,1500,1100,810,720 cm- 1(Characteristics of 1,2,3-triazole system],1H-NMR (400MHz, DMSO-d6) spectrum of((1-(1-(4-tri fluoro phenyl-2-yl) methyl)-1H-1,2,3-triazol-4-yl)methyl)-3,3-di(1H-pyrrol-2-yl] indo line -2-one(7g), was recorded in DMSO-d6 showed the signals at 8 PPm 7.0-7.50(m,9H,C6H4and C6H5 rings),5.90- 6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2-flanked between indolone nucleus and 1,2,3-triazole system), 2.35 (s, 2H, CH2- flanked between1,2,3-triazole system and 4-tri fluoro phenyl ring). 7.42(s,H,1,2,3-triazole ring). Anal. Caled.(%) for C31H33N6O: C:66.18,H:5.91,N:14.94, found C:33.05,H:3.50,N:12.52.
Spectral data for the Compound-7h:
1-((1-(4-nitrophenyl-2-yl)methyl)-1H-1,2,3-triazol-4' yl)methyl)-3,3-di(1H-pyrrol-2-yl) indoline-2-one(7h), Yield 70%, M.P-154-1560C,IR-(KBr) 2861 and 2841 cm-1(Aliphatic CH str.),1743-1691 cm-1(C=O of indoline 2,3-dione) and 1294 cm-1 (C-N str. of indoline 2,3-dione), 3100,2900,1620 ,1420,1000,850,800 cm-1 (Characteristics of 4-nitro phenyl signals), 3500, 1600, 1400,720cm-1(Characteristics of pyrrol signals),3030,1500,1100,810,720 cm-1 (Characteristics of1.2.3- triazole system),1H-NMR (400MHz, DMSO-d6) spectrum of(( 1-(1-(4-nitro phenyl-2-yl)methyl)-1H-1,2,3-triazol'4- yl)methyl)-3,3-di(1H-pyrrol-2-yl) indoline -2-one (7h), was recorded in DMSO-d6 showed the signals at 8 PPm7.0- 7.50(m,9H,C6H4and C6H5 rings),5.90-6.95(m,6H,bis-pyrrol ring),2.35(s,2H,CH2-flanked between indolone nucleus and 1.2.3- triazole system), 2.35 (s, 2H, CH2-flanked between1,2,3- triazole system and4-nitrophenyl ring). 7.42(s,H,1,2,3-triazole ring). Anal. Caled.(%) for C30H33N7O3: C:66.77,H:6.16,N:18.17, found C:33.54,H:3.03,N:16.05.
Preparation of Ag-NPs solution
Silver nanoparticlessolution was prepared using simple methodology by chemical reduction method reported, [24] using solution of AgNO3 and trisodium citrate was added with heating under magnetic stirring, then the solution turned to yellow colour. To confirm the forMicrobial Experimentationion of silver nanoparticles in this solution, we carried out an UV-visible absorption study and TEM imaging. In Fig. 1, a strong characteristic absorption peak around 400 nm is noted for the silver nanoparticles in the solution due to strong but broad surface plasmon peak has been well documented for various Ag-NPs size [25-27].
Transmission Electron Microscopy (TEM) images of the Ag- NPs solution which showed different size of Ag-NPswere recorded using a Zeiss Electron Microscope 10, operating at power 60 kV. TEM samples were prepared by dispersing 2- 3 drops of Ag-NPs solution on copper grid and dried at room temperature after removal of excess solution using a filter paper. A solution of compounds 7b,7a,7c,7h,7g,7f, and 7d were stirred with Ag-NPs solution, the residue products obtained in nano form were confirmed by TEM which showed different size of nano particles.
Microbial Experimentation
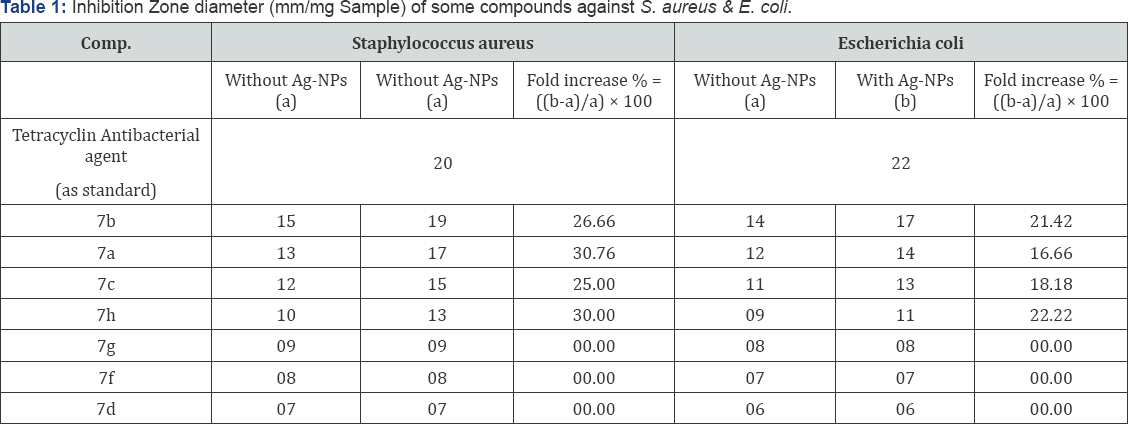
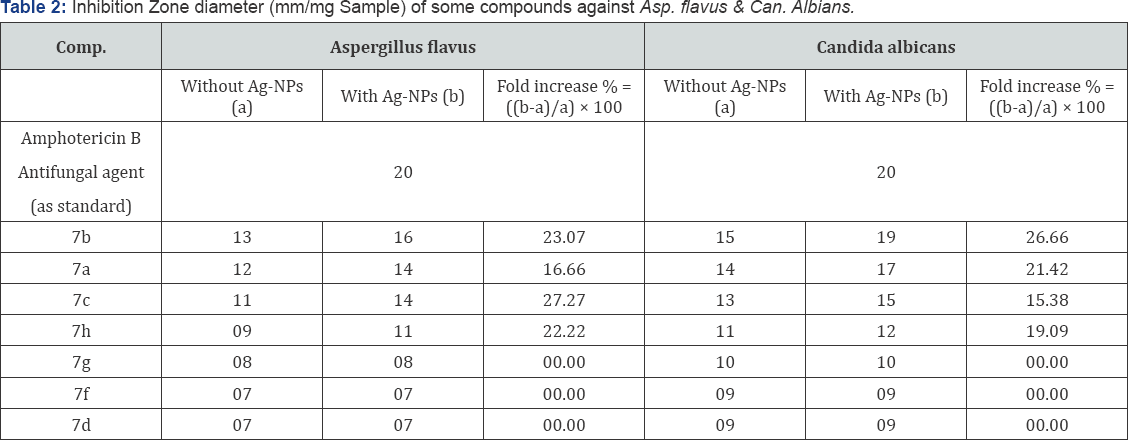
Microbial investigations were done to find the effect of some newly synthesized compounds against Gram +ve)Staphylococcus aureus ATCC (12600) and (Gram -ve) bacteria Escherichia coli ATCC (11775), in addition to their antifungal activity against Aspergillus flavus and Candida albicans with or without silver nanoparticles (Ag-NPs) solution. The preliminary studies of the biological assay were performed according to the agar diffusion method [28-31] at a concentration (25mg/mL) using DMSO as solvents. The results of the in vitro antimicrobial activity were recorded as average diameter of inhibition zone in mm, are given in Tables 1 & 2.
Anti bacterial activity:
The anti bacterial activity of 7b,7a,7c,7h were increased in presence of Ag-NPs solution against both test strains. There is no enhancing effect of 7f,7d,7g on the anti bacterial activities against Staphylococcus aureus and Escherichia coli. The highest fold increases in area were observed for 7b, 7a,7c and 7h in presence of Ag-NPs against both strains.
Anti fungal activity:
The anti fungal activity of 7b,7a,7c,7h were increased in presence of Ag-NPs solution against Aspergillus flavus. There is no enhancing effect of 7f,7d,7g on the anti fungal activities against Candida albicans. The highest fold increases in area were observed for 7b, 7a,7c and 7h in presence of Ag-NPs against both strains.
Conclusion
The current study involved the design and synthesis of new heterocycles based on Bis-pyrrol indoline-2-ones moiety using simple synthetic route to evaluate their antimicrobial and antifungal activities, and study the effect of silver nanoparticles solution on their biological activities. Based on Bis-pyrrol indoline-2-ones triazole. Similar trends were noticed in anti bacterial and anti fungal studies of newly synthesized bis- pyrazoles in presence and absence ofAg-NPs.
Acknowledgement
One of the authors Dr. B. Saritha thank the authorities of S.K. University for providing necessary facilities for carrying out the work. Dept. Of Chemistry, and also the University grants commission for providing financial support (PDFW) Scheme 1.
References
- A Pace, P Pierro (2009) The new era of 1,2,4-oxadiazoles. Org Biomol Chem 7: 4337.
- C Nájera, JM Sansano (2009) 1,3-Dipolar cycloadditions: applications to the synthesis of antiviral agents. Org Biomol Chem 7: 4567.
- PP Gadhave, NS Dighe, SR Pattan, P Deotarse, DS Musmade, et al. (2010) Synthetic and pharmacological profiles of coumarins: A review. Ann Biol Res
- HC Kolb, MG Finn, KB Sharpless (2004) Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem lnt Ed Engl 40(11): 2004-2021.
- AD Moorhouse, JE Moses (2008) Efficient Conversion of AroMicrobial Experimentationic Amines into Azides: A One-Pot Synthesis of Triazole Linkages. Chem Med Chem 3: 715.
- JF Lutz, Z Zarafshani (2008) Efficient construction of therapeutics, bioconjugates, bioMicrobial Experimentationerials and bioactive surfaces using azide-alkyne "click” chemistry. Adv Drug Delivery Rev 60(9):958-70.
- R Huisgen, G Szeimies, L Moebius (1965) 1.3-Dipolare Cycloadditionen, XXIII. Einige Beobachtungen zur Addition organischer Azide an CC- Dreifachbindungen. Chem Ber 98(12):4014-4021.
- CW Torn0e, C Christensen, M Meldal (2002) Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J Org Chem 67(9): 3057-3064.
- VV Rostovtsev, LG Green, VV Fokin, KB Sharpless (2002) A Stepwise Huisgen Cycloaddition Process: Copper (l)-Catalyzed Regioselective "Ligation” of Azides and Terminal Alkynes. Angew Chem lnt Ed 41(14): 2596-2599.
- VD Bock, H Hiemstra, dJ H van Maarseveen (2006) CuI-Catalyzed Alkyne-Azide "Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur J Org Chem 1: 51-68
- F Alonso, G Radivoy, M Yus (2010) Efficiency in chemistry: from hydrogen autotransfer to multicomponent catalysis. Russ Chem Bull Int Ed 14(3): 411-424.
- F Alonso, C Vitale, G Radivoy, M Yus (2011) Multicomponent Click Synthesis of 1,2,3-Triazoles from Epoxides in Water Catalyzed by Copper Nanoparticles on Activated Carbon. J Org Chem 76(20): 83948405.
- F Alonso, Y Moglie, G Radivoy, M Yus (2010) Multicomponent Synthesis of 1,2,3-Triazoles in Water Catalyzed by Copper Nanoparticles on Activated Carbon. Adv Synth Catal 352: 3208.
- T Nishizawa, S Gruschow, EJ Don-Hema, C NishizawaHarada, DH Sherman J, et al. (2006) EnzyMicrobial Experimentationic Assembly of the Bis-Indole Core of Rebeccamycin. Am Chem Soc 128: 724.
- KC Joshi, VN Pathak, SK Jain (1980) Studies of potential organo- fluorine antibacterial agents. Part 5: Synthesis and antibacterial activity of some new fluorine-containing indole-2,3-dione derivatives. Pharmazie 35(11): 677-679.
- A Kamal, MNA Khan, K Srinivasa Reddy, YVV Srikanth, SK Ahmed, et al. (2002) An efficient synthesis of bis(indolyl)methanes and evaluation of their antimicrobial activities. 24(2): 559-565.
- DA Klumpp, KY Yeung, GKS Prakash, GA Olah (1998) Preparation of3,3- Diaryloxindoles by Superacid-Induced Condensations of Isatins and AroMicrobial Experimentationics with a Combinatorial Approach. J Org Chem 59: 4481_4484.
- Ahmed Kamal, M Naseer A Khan, K Srinivasa Redd (2007) Al(OTf)3 as a highly efficient catalyst for the rapid acetylation of alcohols, phenols and thiophenols under solvent-free conditions.
- Welton (1999) Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem Rev 99(8):2071-2084.
- A Paczal, A Kotschy, Monatsh (2007) Asymmetric Synthesis in Ionic Liquids. Chem 138(11): 1115-1123.
- R Sheldon (2001) Catalytic reactions in ionic liquids. Chem Commun 23: 2399-2407.
- SD Riyaz, A Naidu, PK Dubey (2010) Novel and thermally stable ionic liquid (TBA acetate) for domino reaction: Synthesis of spirooxindoles Via new mechanistic way. Syn Comm 53: 1442-1447.
- Yakutik IM, Shevchenko GP, Rakhmanov SK (2004) The ForMicrobial Experimentationion of Mono Disperse Spherical Silver Particles. P. 175.
- Herbert M Zerth, Nicholas M Leonard, Ram S Mohan (2003) Synthesis of Homoallyl Ethers via Allylation of Acetals in Ionic Liquids Catalyzed by Trimethylsilyl Trifluoromethanesulfonate 5: 55-57.
- Henglein A (1993) Physicochemical properties of small metal particles in solution: "microelectrode” reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J Phys Chem 97: 5457-5471.
- Yakutik IM, Shevchenko GP, Rakhmanov SK (2004) The ForMicrobial Experimentationion of Mono Disperse Spherical Silver Particles,Colloids and Surfaces A:Physicochemical and Engineering Aspects. p. 175.
- Sastry M, Mayya KS (1999) Long-Term Stability of Self-Assembled Monolayers of an AroMicrobial Experimentationic Bifunctional Molecule during Adsorption of Silver Colloidal Particles. K Colloid Surf A 15(19): 6587-6590.
- Jawetz E, Melnick JL, Adelberg EA (1947) Review of Medical Microbiology; Lang Medical Publication: Los Altos, California.
- Grayer J, R Harbone (1994) Balanites aegyptiaca Oil Synthesized Iron Oxide Nanoparticles: Characterization and Antibacterial Activity. JB Phytochemistry 37: 19-42.
- Muanza DN, Kim BW, Euler KL, Williams L (1994) Antibacterial and Antifungal Activities of Nine Medicinal Plants from Zaire. Pharm Biol 32(4): 337_345.
- Irobi O, N Moo-Young, M Anderson (1996) Antimicrobial activity of bark extracts of Bridelia ferruginea (Euphorbiaceae). WA Pharm Biol 43(3): 185-190.






























