Phytochemical Profiling and Antifungal Activity m of Essential Oil and Rhizome Extracts of Curcuma Amada Roxb
Phytochemical Profiling and Antifungal Activity m of Essential Oil and Rhizome Extracts of Curcuma Amada Roxb
1Department of Biochemistry, G B Pant University of Agriculture and Technology, India
2Department of Chemistry, G B Pant University of Agriculture and Technology, India
Submission: September 21, 2017; Published: September 26, 2017
*Corresponding author: Om Prakash, Department of Chemistry, College of Basic Sciences and Humanities, G B Pant University of Agriculture and Technology, Pantnagar-263 145, US Nagar, Uttarakhand, India, Email: oporgchem@gmail.com
How to cite this article: Anita T, O Prakash, H Punetha, A K Pant. Phytochemical Profiling and Antifungal Activity of Essential Oil and Rhizome Extracts of Curcuma Amada Roxb. Organic & Medicinal Chem IJ. 2017; 4(1): 555627. DOI: 10.19080/OMCIJ.2017.04.555627.
Abstract
Curcuma amada (Zingiberaceae), commonly used as spice, and is also associated with multiple health benefits. In the present study, petroleum ether, cyclohexane, ethyl acetate, chloroform, acetone and methanol extracts of dried rhizome of C. amada prepared by solvent extraction were analyzed for quantitative and qualitative phytochemical profile and antifungal assay. In vitro antifungal activity of all extracts was determined by using agar well diffusion method against four fungi (Rhizoctonia solani, Sclerotium rolfsii, Collectotricum falcatum and Sclerotenia solani). The essential oil and extracts were found to active against the tested fungi in dose dependent manner by inhibiting the fungul mycelia growth. The essential oil of C. amada exhibited higher activity in comparison to extracts against all the fungi. Maximum inhibition of fungul growth was recorded for R. solani (80.93%) and S. solani (80.90%).
Keywords: Curcuma amda; Extracts; Essential oil; Phytochemicals; Antifungal activity
Research Article
The genus Curcuma belonging to the family Zingiberaceae has a widespread occurrence in the tropical Asia and Australia. This genus, comprises of more than 80 species of rhizomatous herbs including Curcuma amada, Curcuma longa, Curcuma zedoaria, Curcuma aromatica, is widely used in traditional systems of medicines such as Ayurveda, Siddha, Unani, Homeopathy and Naturopathy. In India, it is cultivated in innumerable agro- ecological situations right from the coastal areas to elevations as high as 1880m in the tropics and the sub-tropics of the country [1-3]. Curcuma amada Roxb. It is commonly known as Amada or 'Amahaldi' or 'mango ginger' due to the raw mango-like aroma of the rhizome. Curcuma amada Roxb.
Is a rhizomatous aromatic herb with a leafy tuft and 6090cm in height? Leaves are long, petiolate, oblonglanceolate, tapering at both ends, glabrous and green on both sides. Flowers are white or pale yellow, arranged in spikes in the centre of tuft of the leaves. Lip is semi-elliptic, yellow, 3-lobbed with the mid lobe emarginated [4]. Curcuma amada possess antifungal, antiinflammatory, anticancer and anti hyperglyceridemic properties [5-7]. Rhizomes of Curcuma amada Roxb. Used for the manufacture of oleoresin and essential oil [8]. Its rhizomes essential oil containing β-myrcene, β-pinene, α-pinene, ocimene, ar-curcumene, linalool, linalyl acetate, camphor and safrole [9,10]. Based on the above facts it is imperative to investigate the qualitative phytochemical variations and antifungal activity in various extracts and essential oil.
Material and Methods
Plant material
Air dried rhizomes of C. amada were collected from the local agricultural field of District Udham singh nagar Uttarakhand, India and identified by Dr. D.S. Rawat (plant taxonomist), G.B.Pant University of Agriculture and Technology, Pantnagar. Rhizomes were washed thoroughly to remove adhering material and shade dried at room temperature and was further ground by means of an electrical blender to fine powder.
Isolation of the essential oil
The fresh rhizomes were hydro distilled for using a Clevenger type apparatus for 8h. The oil was extracted with the help of dichloromethane followed by drying over anhydrous Na2SO4. The yield of oil was found about 0.52% (w/v).
Preparation of plant extracts
Plant extracts were prepared in six different organic solvents (Petroleum ether, cyclohexane, ethyl acetate, chloroform, acetone and methanol) using solvent extraction [11]. Rhizome powder (300 g) was extracted in 150 ml of each solvent separately using Soxhlet extractor over water bath for 8h. The extracts were concentrated using vacuum rotatory evaporator at 45±5°C.
Phytochemical screening
Qualitative and quantitative analysis bioassay for total phenol [12], flavanols and orthodihydroxy phenols [13], phytochemical screening of all the six extracts for presence of alkaloids, tannin, anthraquinone, glycosides, reducing sugar, saponins, flavonoids, terpenoids, caumarine, emodins, anthocyanin, betacyanin was carried out according to standard method reported earlier [14-16].
Antifungal activity
The antifungal activity of different solvent extracts was determined by agar well diffusion method [17,18]. Four phytopathogenic fungi, Rhizoctonia solani, Sclerotium rolfsii, Colletotricum falcatum and Sclerotenia solani were maintained and grown on potato dextrose agar medium in order to study antifungal activity of essential oil and various extracts having different polarity.
Sterilized petri plates of 90 mm diameter were used for pouring of medium. In each petri plate about 20 ml sterilized melted medium was aseptically poured near burner flame in a sterilized laminar air flow chamber The medium in the plates were centrally inoculated by placing a 5 mm mycelial disc which was cut from the margin of 5 days old culture of the test fungus. Sterilized four filter paper disc were placed in a sterilized petri plates and different concentration of the extracts were added with the help of sterilized micropipette on each filter paper disc. The plates were sealed with paraflim immediately. Inoculated petri plates were incubated at 26+1°C in a BOD incubator The growth of the fungus was measured in mm at an interval of 24 hours (18).Percent inhibition of growth was calculated by using the following formula:
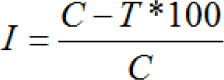
Where, I =lnhibition percentage, C = Colony radius in check (mm) T = colony radius in treatments (mm)
Results and Discussion
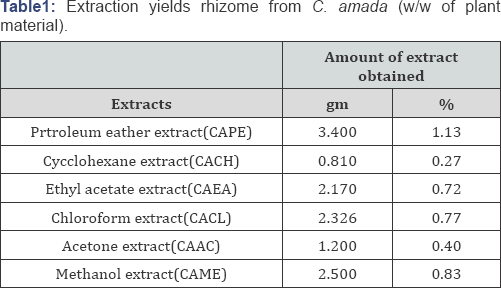
In present study, seeds of C. amada were extracted with six solvents with different polarity (petroleum ether, cyclohexane, ethyl acetate, chloroform, acetone and methanol) using Soxhlet apparatus. The yield of extracts mentioned in (Table 1). All the extracts were screened quantitatively in terms of their total phenols, flavonols and orthodihydroxy phenolic content with the help of their respective calibration curves (Figure 1). CAPE contained 91mg/100mg total phenols more than CAAC, CAEA, CAME, CACCL, CACH. CAME contained 26.31mg/100mg ortho dihydric phenolic contents more than CAPE, CAAC, CACL, CACH, CAEA. The flavonols content was observed more in CACH (75mg/100mg). The values are represented in catechol equivalent. The differences in the antioxidant activity of different extracts of C. amada may be possibly due to the different biochemical make up of the extracts in terms of phenols, flavonols and orthodihydroxy compounds and their concentration in the extracts.

The phytochemicals such as phenolics, flavanoid have been reported to reduce the oxidative peroxidation of lipids by possessing antioxidant activity [19-21]. The chemical composition and in vitro antioxidant potential of essential oil and rhizome extracts of Curcuma amada Roxb was reported in which essential oil was found to possess p myrcene over 40% as a major constituent (10). It has been reported by many workers all over the world that there exist a direct correlation among phenolic contents and antioxidant activity [22,23] (Figure 1).
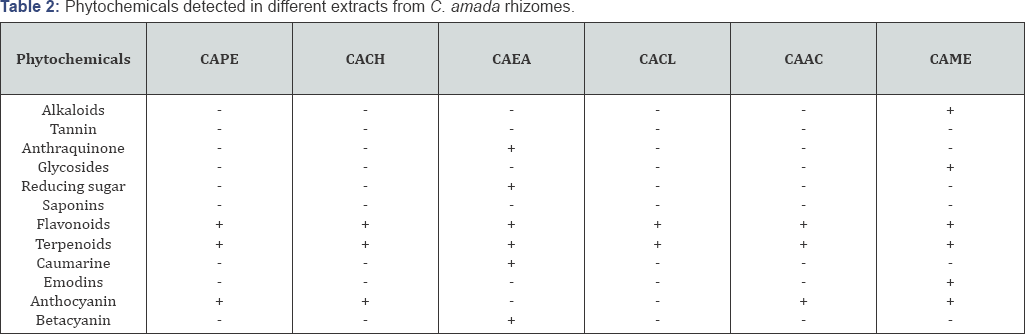
For qualitative secondary metabolite profiling twelve phytochemicals viz. alkaloids, tannin, anthraquinone, glycosides, reducing sugar, saponins, flavonoids, terpenoids, caumarine, emodins, anthocyanin, and betacyanin were analyzed. The study revealed that alkaloids was present only in CAME. Tannin and saponins could not be detected in any extracts. Anthraquinone could be detected only present in CAEA. Glycosides and emodins were present in CAME. Betacyanin, caumarine and reducing sugars were present in CAEA. Flavonoids, terpenoids were present in all extract. Anthocyanin was present in CAPE, CACH, CAAC and CAME (Table 2).
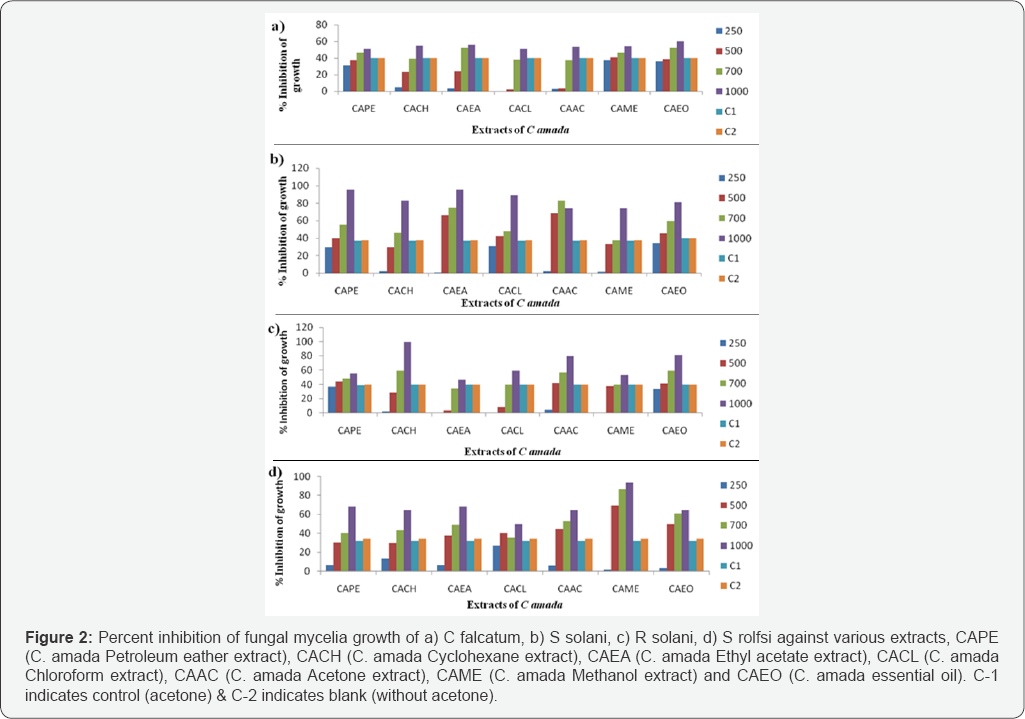
The rhizome essential oil and all the extracts were found active against the tested fungi in dose dependent manner by inhibiting the fungal mycelia growth. All the extracts exhibited good to moderate activity against C. falcatum but the maximum inhibition dose recorded for CAEA (55.90%) at 1000ppm of dose level at 72 hrs. The other extracts inhibited 54.15-50.95% of mycelia growth in order of CACH (54.75%) >CAME (54.15%) >CAAC (53.38%) >CACL (51.05%) >CAPE (50.95%) respectively at the same dose level (Figure 2). Against S. solani all the extracts exhibited well to moderate activity but the maximum inhibition dose recorded for CAPE and CAEA (95.57%) at 1000ppm of dose level at 72 hrs. The other extracts inhibited 88.59 -73.89% of mycelia growth in order of CACL (88.59%) > CACH (82.86%) >CAME (73.95%) >CAAC (73.89%) respectively at 1000ppm of dose level at 72 hrs (Figure 2). CACH was found to be most effective against R. solani, it exhibited 100% growth at 1000ppm of dose level at 72 hrs.
The other extracts inhibited 79.78% - 46.70% of mycelia growth in order of CAAC (79.78%) > CACL (59.18%) > CAPE (55.66%) > CAME (53.40%) >CAEA (46.70%) respectively at 1000ppm of dose level at 72 hrs (Figure 2). Against S. rolfsii the extracts exhibited good antifungal activity but the maximum inhibition dose was recorded for CAME (93.25%) at 1000ppm of dose level at 96 hrs. The other extracts inhibited 68.00 - 49.34% of mycelia growth in order of CAPE (68.00%) > CAME (64.45%)>CACH & CACL (64.43%) >CAEA (49.34%) respectively at 1000ppm of dose level at 96 hrs As per the study the essential oil and all the extracts were found to active against the tested fungi (Rhizoctonia solani, Sclerotium rolfsii, Collectotricum falcatum and Sclerotenia solani) in dose dependent manner by inhibiting the fungal mycelia growth.
The essential oil of C. amada exhibited higher activity in comparison to extracts against all the fungi. The volatile oil from mango ginger rhizomes has antifungal in nature and it has been reported that myrcene and pinene possess antifungal activity against the wide range of fungi, viz. Curvularia palliscens, Aspergillus niger, A. terreus, Fusarium moniliforme and F. falcatum [24]. The major constituents of the essential oil have been reported by our group (10) also possess β-myrcene and β-pinene as the major constituent. Thus the present result of essential oil for possessing antifungal activity against tested pathogenic fungi was justified by the results reported earlier (Figure 2).
Conclusion
With the increased resistance towards synthetic drugs in phytopathogenic fungi, plant products may provide a better alternative to cure as well as prevent the infections caused by them. The present study revealed significant antifungal potential of Curcuma amada along with providing an easy, economic and less polluting way to extract out target bioactive molecules. However further investigations regarding the isolation of individual component from most active extracts may help in offering the natural alternative to treat infections caused by investigated fungus.
Acknowledgement
We are thankful to University Grants Commission (UGC), New Delhi. Thanks are due to the department of Plant pathology bio control lab, G B Pant University of Agriculture and Technology Pantnagar for antifungal activity determination.
References
- Asolkar LV, Kakkar KK, Chakre OJ (1992) Second Supplement to Glossary of Indian Medicinal Plants with Active Principles Part I. Publications and Informations Directorate (CSIR, New Delhi).
- Ghani A (1998) Medicinal plants of Bangladesh Chemical constituents and use. Asiatic Society 86: 290-291.
- Hussain A, Virmani, OP, Popli SP (1992) Dictionary of Indian medicinal plants. Director Central lnstitute of Medicinal and Aromatic Plants Lucknow 161-162.
- Warrier PK, Nambiar VPK, Ramankutty C (1994) In Indian medicinal plants compendium of 500 species. (Chennai Orient Longman Pvt Ltd) pp: 106.
- Ghosh SB, Gupta S, Chandra AK (1980) Antifungal activity in rhizomes of Curcuma amada Roxb. Indian journal of experimental biology 18: 174-176.
- Mujumdar AM, Naik DG, Dandge CN, Puntambekar HM (2000) Antiinflammatory activity of Curcuma amada Roxb in albino rats. Indian J Pharmacol p. 32.
- Rao BL, Dhar AK, Taneja SC, Mehra V (2005) β-myrcene rich Curcuma amada Roxb. A new introduction for Jammu north Indian plains Paper presented in 1st Jammu & Kashmir State. Science Congress 7-9.
- Gupta VK (2001) The wealth of India First supplemented series (Raw materials). New Delhi Council of Scientific and Industrial Research Pusa 2: 259-260.
- Chopra RN, Nayar SL, Chopra IC (1980) Glossary of Indian Medicinal Plants. CSIR New Delhi.
- Tamta A, Prakash O, Punetha H, Pant AK (2016) Chemical composition and in vitro antioxidant potential of essential oil and rhizome extracts of Curcuma amada Roxb. Cogent Chemistry 2: 1168067.
- Turner C (2006) Overview of Modern Extraction Techniques for food and agricultural samples in Modern extraction techniques. Food and agricultural samples C Turner Ed ACS symposium series 926 American Chemical Society Washington DC 3-19.
- Shetty K, Curtis O F, Levin RE, Wikowsky R, Ang W (1995) Prevention of Verification associated with in vitro shoot culture of oregano (Origanum vulgare) by Pseudomonasspp. Journal of plant phy 147: 447-451.
- Mahadevan A, Sridhar R (1986) Methods in physiological plant pathology. (3rd end) Sivakami Publication Chennai 183-184.
- Rauf A, Uddin G, Ali M, Muhammed N, Gul S (2013) Phyto chemical screening and antioxidant activity of Pakistani medicinal plants. Wud pecker Journal of Med Plants 2(1): 001-006.
- Savithramma N, Lingarao M, Suhrulatha D (2011) Screening of medicinal plant for secondary metabolites. Middle East J Sci Re 8(3): 579-584.
- Uddin G, Rauf A, Rehman TU, Qaisar M (2011) Phyto chemical Screening of Pistacia chinensis var. integerrima. Middle East J Sci Res 7(5): 707711.
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME et al. (2012) Multi drug resistant extensively drug resistant and pan drug resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance . Clin Microbiol Infect 18(3): 268-281
- Bauer AW, Kirby WMM, Sheriss IC, Turck M (1966) Antibiotic Susceptibility testing by standardized single method. J Clin Pathol 45: 493-496.
- Martin FR, Frutos MJ, Perez Alvarez JA, Martinez Sanchez F, Del Rio JA (2002) Flavanoids as nutra ceuticals: structural related antioxidant properties and their role on ascorbic acid preservation. In Atta-Ur- Rahman (editor) Studies in natural products chemistry Elsevier Science Amsterdam p. 324.
- Neogi NC, Garg RD, Rathore R (1970) Preliminary pharmacological studies on achyranthine. Ind J Pharm 32: 43-46.
- Skerget M, Kotnik P, Hadolin M, Hras AR, Simonic M et al. (2005) Proantho cyanidins flavones and flavonols in some plant materials and their antioxidant activities. Food chemistry 89: 191-198.
- Sethi S, Prakash O, Pant AK (2015) Essential oil composition antioxidant assay and antifungal activity of essential oil and various extracts of Alpinia alughas (Retz) Roscoe leaves. Cogent Chemistry 1: 1-12.
- Sethi S, Prakash O, Punetha H, Pant AK (2016) Antioxidant assay and antifungal activity of essential oil and various extracts from Alpinia allughas Roscoea: A potent Zingiberaceous Herb. J Essen Oil Bear Pl p. 19: 358.
- Singh G, Singh OP, Maurya S (2002) Chemical and Biocidal Investigations on Essential Oils of some Indian Curcuma Species. Prog Crystal Growth Charact p. 45: 75-81.






























