Electron Transfer Process between C60 Fullerene and Memberane Cells of Salmonella Typhimurium; a Theoretical Study
Narges Zolfaghar1 and Avat (Arman) Taherpour2*
1Organic Chemistry Department, Chemistry Faculty, Razi University, Iran
2Medical Biology Research Center, Kermanshah University of Medical Sciences, Iran
Submission: September 16, 2017; Published: September 21, 2017
*Corresponding author: Avat (Arman) Taherpour, Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran; Email: avatarman.taherpour@gmail.com
How to cite this article: Narges Z, Avat (Arman) T. Electron Transfer Process between C60 Fullerene and Memberane Cells of Salmonella Typhimurium; a Theoretical Study. Organic & Medicinal Chem IJ. 2017; 3(5): 555623. DOI: 10.19080/OMCIJ.2017.03.555623.
Abstract
The relationship between the number of carbon atoms and the free energies of electron transfer (AGet) were investigated using the Marcus Theory and Rehm-Weller equation for ET- (electron transfer) and PET-process (Photoelectron Transfer) between fullerenes and the cells wall of Salmonella typhimurium. The calculations are presented for the oxidation potential (Ox.E1) of fullerene (C60). The results were used to calculate the free-energies of electron transfer (ΔGet) of the ET-process for the fullerenes. The first free activation energies of electron transfer and the maximum wave length of the electron transfers, ΔG#et(n) and λet, respectively, were also calculated in this study for the selected conditions in accordance with the Marcus theory.
Keywords: Fullerenes; Salmonella typhimurium; Electrical potential; Electron transfer process; free energies of electron transfer; Rehm-Weller equation; Plank's formula; Marcus Theory
Abbreviations: LPS: Lipo Poly Saccharides; PMF: proton motive force ET: Electron Transfer; LE: Locally Excited; TS: Transition State; QMD: Quantum Molecular Dynamics
Introduction
Salmonella typhimurium is a pathogenic Gram-negative bacteria predominately found in the intestinal lumen. Its toxicity is due to an outer membrane consisting largely of Lipo Poly Saccharides (LPS) which protect the bacteria from the environment [1]. The LPS is made up of an O-antigen, a polysaccharide core, and lipid A, which connects it to the outer membrane. Lipid A is made up of two phosphorylated glucosamines which are attached to fatty acids. These phosphate groups determine bacterial toxicity. Animals carry an enzyme that specifically removes these phosphate groups in an attempt to protect themselves from these pathogens. [1] The O-antigen, being on the outermost part of the LPS complex is responsible for the host immune response. S. typhimurium has the ability to undergo acetylation of this O-antigen, which changes its conformation, and makes it difficult for antibodies to recognize [1].
The two basically forms of metabolic energy in microorganisms have introduced as: a) the ions (such as proton) gradients provide the electrochemical energy for cell of microorganisms, and b) the energy-rich phosphate bonds such as ATP molecule. A cell of bacteria includes an organized cytoplasm and the organization of the proteins particularly for those intricate and important morphogenetic processes is for the cell operations such as morphogenetic processes [2] The ApH and the A¥ (trans-membrane chemical proton gradient and trans-membrane electric potential, respectively) were constructed by the producing Proton Motive Force (PMF) [3] .
The different empty fullerenes (Cn) with various number of carbon atoms "n" such as C60, C70, C76, C82 and C86, have been obtained [4-6]. Because of the different number of the carbon atoms in their structures these molecules show different chemical, physical and mechanical properties. The compressive mechanical behaviors of the empty fullerenes Cn (n = 20, 60, 80, and 180) were investigated with using QMD (Quantum Molecular Dynamics) technique by Shen [4] the interesting stability of molecular allotropes C60 and C70 After the discovery of C60 peapods [5] the aligned structure of encapsulated molecules has been studied as a new type of hybrid material due to the molecule-molecule interactions. Since the early 1990s, the electrochemical properties of the C60 were studied when these materials first became available in macroscopic quantities.
In 2003, for the first time, the hypothesis of fullerenes acting as electron drainer which can disrupt the respiratory chain with electron leakage by Mashino et al. [6] The report was focused on positively charged derivatives, however the behind mechanism of interactions seems to have common points. [6] This study elaborates on the electron transfer process between C60 fullerene and the membrane cells of Salmonella typhimurium. The relationship of the free energies of electron transfer (AGet) between the cell wall of Salmonella typhimurium bacteria with C60 fullerene and ΔG#et(n) of the ET-process, on the basis of the oxidation potentials (Ox. E1) of the C60 fullerene as assessed by applying the Rehm-Weller equation and Marcus Theory. [6] In this study, were also calculated the activate free energies of electron transfer and the maximum wave length of the electron transfers (ΔG#et(n) and λet), applying Marcus theory on the basis of the oxidation potentials of the fullerenes C60 to predict the data of the ET-process between the membranes of the membrane cells of Salmonella typhimurium and C60 fullerene.
Methods, Results and Discussion
The LUMO orbital of C60 can accept up to six electrons (6e) to form C606-, but the position of the HOMO does not allow for hole- doping under the usual reported electrochemical conditions. Haufler et al. [7] have demonstrated the reduction C60 to C601- and C602- electrochemically in CH2Cl2 media. Echegoyen et al. [8] electrochemically reduced C60 in six reversible steps for -0.97V vs. Fc/Fc+. The irreversible electrochemical and structural reorganization of solid fullerenes in acetonitrile was reported by Bard et al. The experimental conditions by investigating highly organized C60 films on HOPG in an aqueous medium was improved by Dunsch et al. [9] the reduction of the tin films induces a morphological change.
All of the mathematical operations have performed using Microsoft Office Excel-2003 and MATLAB-7.4.0(R2007a) programs. Equation 1 has applied to measure the values of Get for the dipolar complexes that have yet to be reported in the literature. The free energy changes between an electron donor (D) and an acceptor (A) estimates by Rehm-Weller equation estimates, as: [10].

In this equation "e" is the unit electrical charge, EDo and EAo are the reduction potentials of the electron donor and acceptor, respectively, ΔE* is the energy of the singlet or triplet excited state and w1 is the work required to bring the donor and acceptor to within the Electron Transfer (ET) distance. If an electrostatic complex constructs before the ET process, the work term in this expression is equal to zero [11-12].
The Marcus theory of ET-process implies rather weak (<0.05eV) electronic coupling between the initial (Locally Excited, LE) and final (Electron Transfer, CT) states and presumes that the TS (Transition State) is close to the crossing point of the LE and CT terms [13]. The value of the ET-rate constant ket is controlled by ΔG#et, which is a function of the reorganization energy (l/4) and electron transfer driving force ΔGet:
ΔG #et = (l/4)(1 + ΔGet /l) 2 (Eq.-2)
The reorganization energy of organic molecules ranges from 0.1-0.3 eV. In this study, was utilized the minimum amount of the reorganization energy [14].
The maximum wavelength (λet) of the electromagnetic photon for the electron transfer process in the dipolar complex was calculated by Planck's formula:
ΔG # et = AE = h.c / X(n) (Eq.-3)
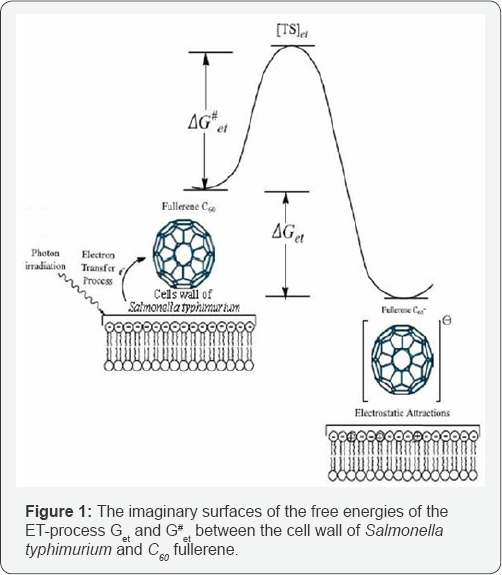
In Figure 1 has shown the imaginary ET-process between the cell wall of Salmonella typhimurium bacteria and C60 fullerene to construct the dipolar complexes by electrostatic attraction after ET and/or PET process. In Figure 2 has demonstrated the imaginary surfaces of the free energies of the ET-process
Get and G#et between the cell wall of Salmonella typhimurium and C60 fullerene. See Table 1 for the calculated data. On the basis of the obtained results from literatures the Ox.E1of C60 and Red E of Salmonella typhimurium bacteria is 1.12 and 0.00154 Volt, respectively [15-18].
The results of the calculations of the ET and/or PET-process and applying the equations 1-4 show that the values of the free-energies of electron transfer (AGet), first free activation energies of electron transfer and the maximum wave length of the electron transfers, ΔG#et(n) and Aet, respectively, were: 27.17, 35.94 kcal mol-1 and 795nm, respectively. It was assumed that the discussed ET-process has also stopped some phenomena related to restriction in bacteria growth by perturbation on the membrane charges of Salmonella typhimurium bacteria cell wall. See equations 1-4, Figures 1 & 2 and Table 1. The calculated data for ET- and/or PET-process between the other fullerenes (C70, C76, C82 and C86) and cell wall of Salmonella typhimurium bacteria were demonstrated in Table 1. In agree with the obtained results the ET process has occurred in lower ΔG#et(n) and bigger λet by increasing the electron population in greater fullerenes. The PET process from Salmonella typhimurium to C60 fullerene occurs at about 795nm, but, for greater fullerenes (like C76 to C86) it will occur in near-lR region.
In this study, the values of the maximum wavelengths (AGet) for the ET-process between the selected fullerenes and the bacteria cell wall in the dipolar complexes by Planck's formula were demonstrated. The photonic energy of the ET-process was also determined by the use of this formula. Most of the Values were found in the range of Visible to near-lR (795-1640nm) range of the electromagnetic spectrum. The Get depends on the G#et value in each state. The values of Get have increased by decreasing of the G#et value in each state. In this study, the photo-electron transfer process was investigated to find medicinal and antibiotic activities for the selected fullerenes by performing the complexes between fullerenes and the bacteria membrane (figures 1 & 2) (Table 1).


Conclusion
The results of this study may be applied in some medical treatment operations, such as irradiation of the appropriate λet to destruct the membrane cells of Salmonella typhimurium bacteria in presence of Cn (n=60, 70, 76, 82 and 86) fullerenes. The results discussed here and the calculated values of Get, G#et and Aet corresponding to the electron transfer process have neither predicted nor reported before. It is supposed that the ET- and/or PET-process between the membrane of Salmonella typhimurium bacteria with Cn (n=60, 70, 76, 82 and 86) fullerenes prepare the conditions to restrict the bacteria (Salmonella typhimurium) growing by perturbation in the cell wall charges of Salmonella typhimurium.
References
- Klaus Winzer, Kim Hardie, Nicola Burgess, Neil Doherty, David Kirke, et al. (2002) LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 148: 909-922.
- J Slauch (1995) Salmonella typhimurium. 63(2): 437-441.
- BI Kanner, DL Gutnick, HR Kaback (1972) Physiological suppression of a transport defect in Escherichia coli mutants deficient in Ca2+, Mg2+- stimulated adenosine triphosphatase. Gutnick J Bacteriol 111: 187.
- H Shen (2007) The compressive mechanical properties of Cn (n=20, 60, 80, 180) and endohedral M@C60 (M=Na, Al, Fe) fullerene molecules. Molecular Physics 105(17-18): 2405.
- LE Rryan, SK Kowand, HM Van Den Elzen (1979) Mechanism of Aminoglycoside Antibiotic Resistance in Anaerobic Bacteria: Clostridium perfringens and Bacteroides fragilis. Antimicrob Agents Chemother 15(3): 7-13.
- T Mashino, D Nishikawa, K Takahashi, N Usui, T Yamori, et al. (2003) Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg Med Chem Lett 13(24): 4395-4377.
- RE Haufler, J Conceicao, LPF Chibante, Y Chai, NE Byrne, et al. (1990) Efficient production of C60 (buckminsterfullerene), C60H36, and the solvated buckide ion. J Phys Chem 94(24): 8634-8636.
- Q Xie, E Perez-Codero, L Echegoyen (1992) Electrochemical detection of C606- and C706-: Enhanced stability of fullerides in solution. J Am Chem Soc 114(10): 3978-3980.
- P Janda, T Krieg L Dunsch (1998) Nanostructuring of Highly Ordered C60 Films by Charge Transfer. Adv Mater 17: 1434.
- LE Rryan, HM Van Den Elzen (1976) Streptomycin Accumulation in Susceptible and Resistant Strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother 9(6): 928-938.
- H Taber, GM Halfenger (1976) Multiple-Aminoglycoside-Resistant Mutants of Bacillus subtilis Deficient in Accumulation of Kanamycin. Antimic. Agen. Chemother 9(2): 251-259.
- E Padan, D Zilberstein and H Rottenberg (1976) The Proton Electrochemical Gradient in Escherichia coli Cells. Eur J Biochem 63: 533.
- HW Kroto, JR Heath, SC O'Brien, RF Curl, RE Smalley, et al. (1985) Cytotoxicity of Pristine C60 Fullerene on Baby Hamster Kidney Cells in Solution. Nature 318: 162.
- HW Kroto (1987) The stability of the fullerenes Cn, with n = 24, 28, 32, 36, 50, 60 and 70. Nature 329: 529.
- RA Marcus (1993) Electron transfer reactions in chemistry. Theory and experiment. Rev Modern Physics 65(3): 599.
- RA Marcus, N Sutin (1985) Electron transfers in chemistry and biology. Biochim Biophys Acta 811: 265.
- D Rehm, A Weller (1970) Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr J Chem 8: 259.
- C Jehoulet, YO Obeng, YT Kim, F Zhou, AJ Bard, et al. (1992) Electrochemistry and Langmuir trough studies of fullerene C60 and C70 films. J Am Chem Soc 114(11): 4237-4247.






























