Electrophilic Fluorination with 1-Fluoro-2,4,6-trimethylpyridinium tetrafluoroborate and NFSI (N-Fluorobenzenesulfonimide) reagents directed towards the synthesis of heterocycles ; Current Trends
Pankaj Sharma, Nutan Sharma, Amit Kumar and SunitaBhagat*
Department of Chemistry, ARSD College, University of Delhi, India
Submission: September 06, 2017; Published: September 19, 2017
*Corresponding author: SunitaBhagat, Department of Chemistry, ARSD College, University of Delhi, Dhaula Kuan, New Delhi-110021, Indias
How to cite this article: Pankaj S, Nutan S, Amit K, SunitaB. Electrophilic Fluorination with 1-Fluoro-2,4,6-trimethylpyridinium tetrafluoroborate and NFSI (N-Fluorobenzenesulfonimide) reagents directed towards the synthesis of heterocycles ; Current Trends. Organic & Medicinal Chem IJ. 2017; 3(4): 555620. DOI: 10.19080/OMCIJ.2017.03.555620
Abstract
The phytochemical screening and the antidiarrhoeal activities of the crude extracts from the leaves Landolphiaowariensis were carried out using standard methods. The results of the phytochemical screening showed that the plant contained alkaloids, saponins, tannins, flavonoids and glycosides. The results of the antidiarrhoeal screening of the crude extracts showed that Landolphiaowariensis exhibited zone of inhibition ranging from 15 mm to 35 mm against test microbes. Ethyl-acetate extract of the Landolphiawariensis being the most active fraction was subjected to column chromatography, leading to the isolation of single compound that showed a high activities (zone of inhibition from 8mm to 32mm) against these causative agents of diarrhoea (Salmonella Typhi, Shigelladysentriaeand Escherichaia Colirespectively). Spectroscopic analysis of the isolated compound using 1HNMR, 13CNMR, IR and GCMS showed that the compound was Cis-9-Octadecenoic acid. The result of this works therefore showed that the extract from the leaves of Landolphiaowariensis could be used for the treatment of diarrhoea and other diseases caused by targeted microorganisms
Keywords: Antidiarrheal; Landolphiaowariensis; Isolation; Cis-9-Octadecenoic Acid
Introduction
The development of fluorination chemistry began more than 100 years ago with the first examples of nucleophilic and electrophilic fluorination reactions being reported in the second half of the 19th century [1,2]. Fluorinated molecules plays a significant role in pharmaceutical/medicinal [3,4] agrochemical [5,6] and material sciences [7] due to the unique properties of fluorine atom [8]. The introduction of fluorine atom can modulate the properties of biologically active compounds, since this may lead to changes in lipophilicity, chemical reactivity, solubility, conformation, ability of hydrogen-bonding [9]. As a result, 30-40% of agrochemicals and 20-25% of pharmaceuticals on the market are estimated to contain fluorine [10].
The efficacy of agrochemicals and pharmaceuticals oftenly enhanced or it is due to the presence of fluorine atom in the structure [11]. Frequently, these type of compounds can be synthesized from small moieties in which fluorine is located at a specific site, or often it is desirable to introduce the element directly in order to obtain the desired chemical composition [12,13]. Electrophilic fluorination is one of the most direct methods for selective introduction of fluorine into organic compounds [14]. In this respect, various reagents for electrophilic fluorination have been developed till now as shown in (Figure 1). Elemental fluorine itself is one of the most powerful reagents [15].
However, fluoroxcompounds [16], such as CF3OF, CF3C(O) OF, CsSO4F, and CH3C(O)OF13 some of which are generated in situ, are exciting reagents for the introduction of fluorine electrophilically into a wide variety of organic compounds [17,18]. Most electrophilic fluorinating reagents are ultimately derived from fluorine gas, the strongest elemental oxidant known, which is synthesized by electrolysis of potassium difluoride in hydrogen fluoride [19]. Electrophilic fluorination reactions with highly oxidizing fluorinating reagents [20] such as fluorine gas, hypofluorites, fluoroxysulfates, and perchloryl fluoride are challenging to perform due to the high reactivity of the reagents. Xenon difluoride was developed as a more stable electrophilic fluorination source, but its high oxidation potential still limits the tolerance of this reagent to functional groups [21].
The development of crystalline, benchtop-stable fluorinating reagents such as N-fluorobis (phenyl) sulfonimide (NFSI) [19] and related analogues [22,23], N-fluoropyridinium salts [24], and 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo-[2.2.2] octane bis(tetrafluoroborate) (Selectfluor, F-TEDABF4) [25] was crucial for the development of selective, functional-group- tolerant fluorination methods. Another class of electrophilic fluorinating reagents with the general structure R2N-F or R3N+-F began to gain popularity. In comparison to the previous reagents, these compounds were stable, safer, milder and less expensive to produce. Furthermore, some of these compounds proved to be as reactive as established reagents in some cases, which were also capable of a degree of selectivity that was previously unattainable [26]. Efforts by Umemoto et al. led to the first isolatable N-fluoropyridinium salts, which had good activity and were amenable to commercial production [27] (Figure 1).
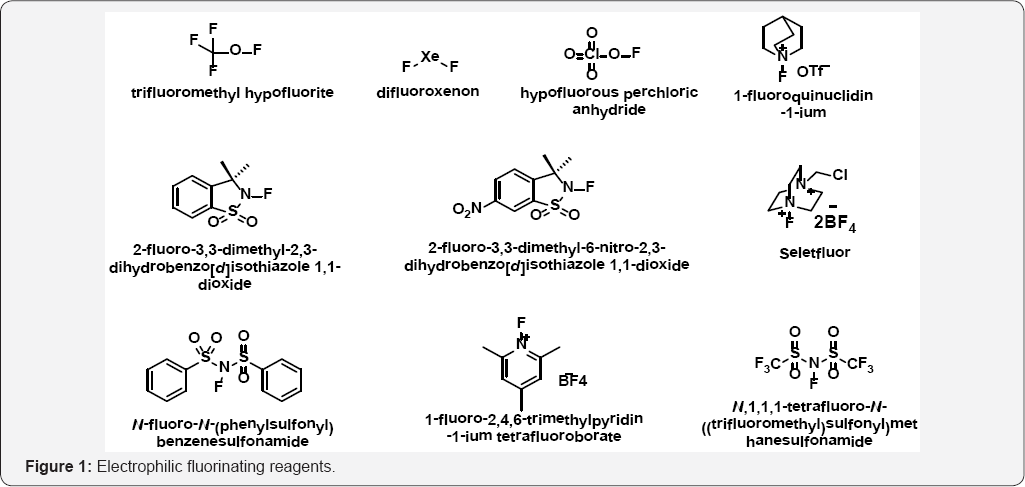
The important role of the counter anion, which influences the reactivity, selectivity, and stability of the reagent, was also demonstrated [28]. Des Marteau and co-workers later reported the discovery of N-fluorobis [(trifluoromethyl) sulfonyl] imide, accessed by the reaction of bis [(trifluoromethyl) ulfonyl]-imide with fluorine gas [29,30]. The synthesis of this compound, which is still one of the most powerful sources of electrophilic fluorine, was a step forward in the identification of a stable source of electrophilic fluorine with desirable physical properties. One major drawback is that it is not commercially available, so that the use of fluorine gas for its preparation in the laboratory is ultimately required.
More recently, the development of the reagent NFSI presented a major advance for electrophilic fluorination, as it is a reliable, mild, stable, and effective source of electrophilic fluorine that lends itself to large-scale synthesis and is commercially available. A number of reagents, each with its advantages and disadvantages, currently exist for the electrophilic incorporation of fluorine and have been the subject of several reviews [31-34]. In the present review, we give a comprehensive overview of the literature reports emphasizing on two potential electrophilic fluorinating reagents 1-Fluoro-2,4,6-trimethylpyridinium tetrafluoroborate and NFSI (N-Fluorobenzenesulfonimide).
Electrophilic Fluorinating Reagents
Fluoropyridinium tetrafluoroborate: (Scheme 1)

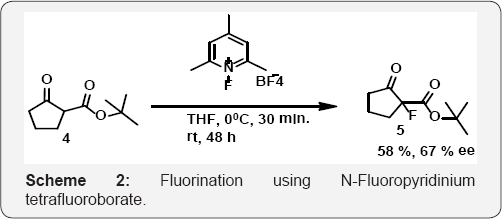
In (Scheme 1), Lagow and co. workers used N-Fluoropyridinium tetrafluoroborate as fluorinating reagent among the N-fluoropyridinium salt series and can react as an electrophilic fluorine source. Exhibiting almost the same reactivity as other related N-fluoropyridinium salts, albeit with a somewhat lower solubility, it has a high fluorine content (103 g kg-1) [35] (Scheme 2).of organic molecules. The C-H activation/oxidative fluorination process allows ortho-fluorination of the aryl ring under microwave irradiation [38] (Scheme 4).
Tomita, K. and Co workers used N-Fluoropyridinium tetrafluoroborate as fluorinating reagent as shown in (Scheme 2). In this case of fluorination of 1-(trimethylsilyloxy) cyclohexene, the yields and/or rates of reaction are not satisfactory, compared to those of the triflateanalog, because of the reduced solubility of fluorinating reagents in CH2Cl2. The same reactions performed in acetonitrile, however, give similar results [36] and efficient enantioselective electrophilic fluorination of β-ketoesters can be achieved. It can be seen that use of NFPY (N-fluoropyridinium tetrafluoroborate) resulted in moderate enantioselectivities. Interestingly, the addition of the electron-poor alcohol HFIP (1,1,1,3,3,3-hexafluoroisopropanol) as additive led to an increase in enantioselectivity to 67% ee [37](Scheme 3).
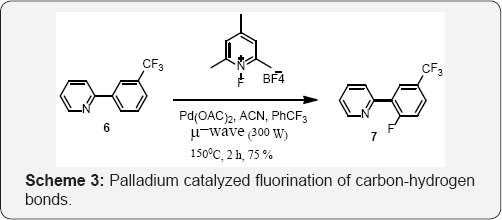
1-fluoro-2,4,6-trimethylpyridin-1-iumtetrafluoroborate was selected as the best electrophilic fluorine donor for palladium- catalyzed fluorination on 2-arylpyridines. In Scheme 3, Takeru Furuya and Co-workers used Palladium catalysed fluorination of organic molecules. The C-H activation/oxidative fluorination process allows ortho-fluorination of the aryl ring under microwave irradiation [38] (Scheme 4).
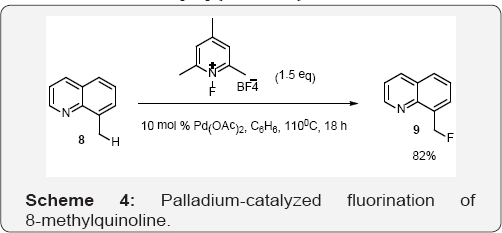
In Scheme 4, Sanford and co workers used Palladium- catalyzed fluorination of 8-methylquinoline. Initial investigations focused on the Pd (OAc)2-catalyzed benzylic fluorination of 8- methylquinoline. This substrate was selected because it undergoes facile quinoline-directed C-H activation at Pd(II) to generate a 6-benzyl-Pd species and, as such, has been shown to serve as an excellent substrate for related Pd-catalyzed C-H activation/oxidative functionalization reactions [39,40]. An initial screen of F+ reagents under thermal reaction conditions (10 mol % of Pd (OAc)2, 110 °C, 13 h, benzene) revealed that several conditions affected the desired benzylic C-H bond fluorination reaction, providing desired compound in modest 9- 36% yields while using different fluorinating reagents [41]. However, N-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate served as a highly effective F+ source affording products in a dramatically improved 82% isolated yield. Importantly, control reactions in the absence of Pd catalyst showed none of the fluorinated product [42].

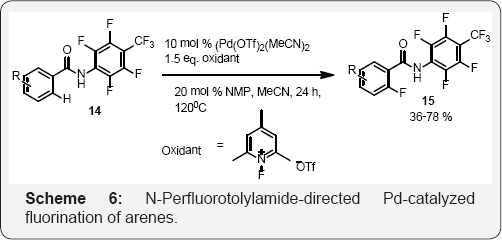
In Scheme 5, experimental investigation concludes a change of rate limiting step using 8-aminoquinoline vs. 8-aminoquinoxaline as directing group by affecting the key Pd (II) to Pd(IV) oxidation step. This finding led to the discovery of a simple ligand capable of lowering the activation energy for the oxidation of Pd. This intriguing mechanistic journey provides new clues into oxidative C-H functionalization reactions. We expect these results will stimulate the development of new C-H functionalization reactions. It is being observed that if 8-aminoquinoline vs. 8-aminoquinoxaline are removed from the reaction mixture the rate of reaction is affected and the overall yield of the reaction affected by 42 %. If the reaction performed without ligand the yield is only 15% and the yields vary with different ligands [43] (Scheme 5) and (Scheme 6).
Double fluorination through two subsequent ortho fluorination events was addressed with a weakly coordinating anionic ortho-directing group N-perfluorotolylamide derived from benzoic acid that allows for rapid displacement of the monofluorinated product by the substrate, thus affording high selectivity for monofluorination (Scheme 6).
Electrophilic fluorination using N-fluoro-N- (phenylsulfonyl) benzene sulphonamide (NFSI):
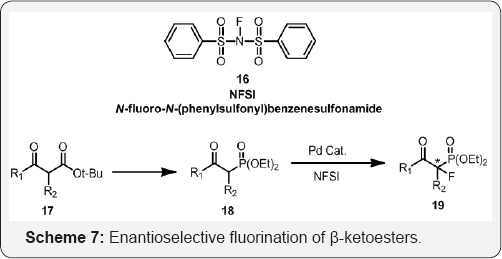

Mikiko Sodeoka co-workers reported a highly efficient catalytic enantioselective fluorination of β-ketoesters (Scheme 7) [44]. In the presence of a catalytic amount of chiral Pd complexes, a variety of β-ketoesters reacted with N-fluorobenzenesulfonimide (NFSI) in an alcoholic solvent such as EtOH to afford the corresponding fluorinated products with excellent enantioselectivity (up to 94% ee). Various substrates including cyclic and acyclic p-ketophosphonates underwent the reaction with N-fluorobenzenesulfonimide (NFSI) in EtOH to give the corresponding fluorinated products in a highly enantioselective manner (94-98% ee). (Schemes 8 & 9) and (Scheme 10)
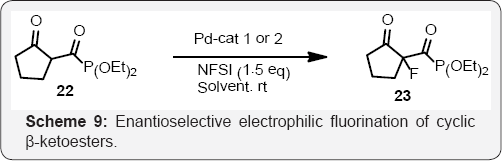
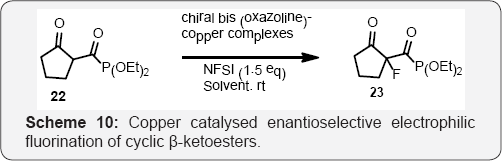
Dominique Cahard and Co workers developed a new efficient catalytic enantioselective electrophilic fluorination of acyclic (Scheme 8) and (Scheme 9) cyclic β-ketoesters is developed [45]. As low as 1 mol% of chiral bis (oxazoline)- copper triflate complexes catalyses the fluorination by means of N-fluorobenzenesulfonimide (NFSI). The use of 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) is crucial for achieving high enantioselectivity [46]. Herein a new efficient catalytic enantioselective electrophilic fluorination of both cyclic and acyclic β-ketoesters by means of chiral bis (oxazoline)-copper complexes leading to enantio enriched fluorinated compounds in high yields and good enantioselectivities (scheme 10). The positive impact on the enantiomeric excess of achiral additives such as HFIP is also reported [47,48] (Scheme 11).

In (Scheme 11), fluorination of Grignard reagents with electrophilic N-fluorinated reagents NFSI is the most reliable method with simple aryl nucleophiles but is narrow in scope due to the basicity and nucleophilicity of the arylmagnesium reagents [49,50]. Through appropriate choice of solvent and reagents, undesired protodemetallation products can be minimized [51]. More recent work by Meng and Li demonstrated the regioselective para fluorination of anilides with PhI(OPiv)2 and hydrogen fluoride/pyridine [52](Scheme 12).
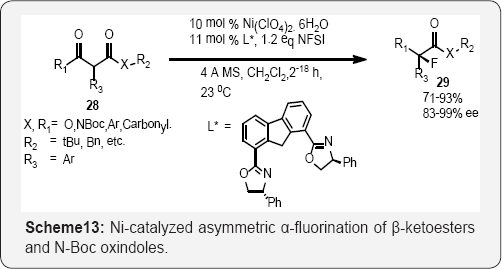

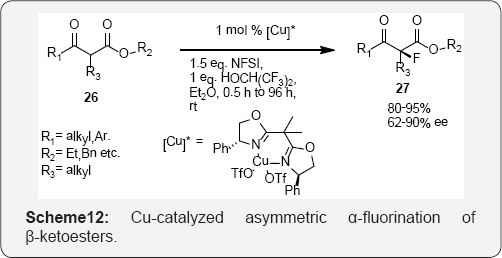
Several methods have exploited the two-point binding of dicarbonyl compounds to chiral lewis acid complexes to control enantioselective fluorination. Asymmetric fluorination of β-ketoesters was achieved with Cu (II)- (Scheme-12) and (Scheme 13) [53]. Several methods have exploited the two-point binding of dicarbonyl compounds to chiral lewis acid complexes to control enantioselective fluorination. Asymmetric fluorination of β-ketoesters was achieved with Ni (II)-BOX complexes (Scheme 13) [54] by Cahard and Shibata/Toru, respectively (Scheme 14).
Chiral bis (imino) bis (phosphine) ruthenium (II) complex by Togni (Scheme 14) [55] and scandium binapthyl phosphate complexes by Inanaga [56]. The Ni-catalyzed reaction, using a 10 mol% catalyst loading, has demonstrated the broadest substrate scope so far, and allows for α-fluorination of a variety of β-ketoesters and N-Boc-protected amides [52] in 71-93% yield and 83-99% ee, respectively. The catalytic enantioselective a-fluorination of a-substituted methyl, tert-butyl malonate was accomplished via chiral lewis acid catalysis with Zn (II) acetate, (R,R)-4,6-dibenzofurandiyl-2,2'-bis(4-phenyloxazoline) ligand, and NFSI. This approach was specifically optimized for the malonate substrate en route to the enantioselective synthesis of fluorinated β-lactams [57,58].

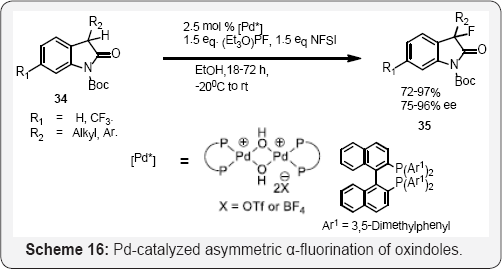

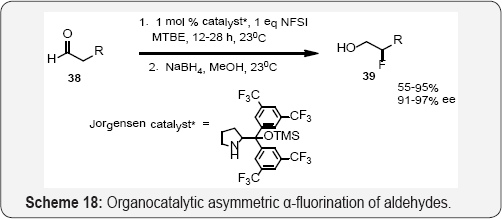
Chiral Pd-BINAP complexes developed by Sodeoka shown in (Scheme 15) and (Scheme 16) catalyzes the enantioselective fluorination of α-ketoesters [59], β-ketoesters (Scheme 11), [60] β-ketophosphonates, [61] oxindoles, [62] and α-ester lactones/lactams [63]. The use of chiral palladium complexes was particularly successful for the α-fluorination of acyclic α-ketoesters, cyclic and acyclic tert-butyl β-ketoester as well as oxindoles α-substituted with an electronically diverse range of aryl and alkylgroups [59-62] (Scheme 17). The aldehyde α-fluorination method described by MacMillan demonstrates a broader substrate scope, (Scheme 17) [64]. These fluorinated compounds are useful for the synthesis of different biological active heterocycles [65]. Jθrgensen and co workers used NFSI and organocatalytic α-fluorination of aldehydes. (Scheme 18) [66] While the method described the utilization of lower loadings of catalyst and electrophilic fluorinating reagent. Branched aldehydes constitute difficult substrates for enantioselective α-fluorination; nonetheless.
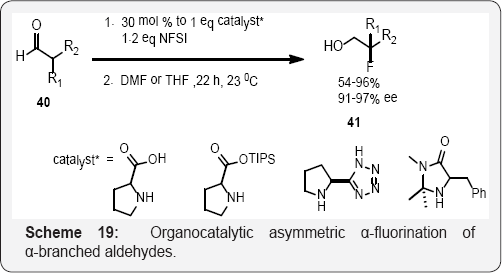
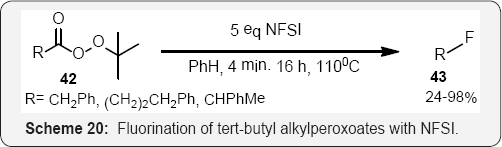
Barbas (Scheme 19) [67] accomplished the enantioselective organocatalytic α-fluorination of aldehydes. Similarly enantioselective α-fluorination of ketones is possible using enaminecatalysis [68]. Barbas reported a promising 98-99% yield and 45-66% ee for this class of substrates. The fluorinated aldehyde products are especially useful for the synthesis of enantiopure β-fluoroamines, which can be obtained by a chiral sulfinyl imine condensation, directed reduction sequence of the enantioenriched fluorinated aldehyde [69] (Scheme 20). The N-F bonds in electrophilic fluorinating reagents have relatively low bond dissociation energies (2.84 eV for N-fluorosultam) [70]. Under photolysis or thermolysis conditions, a variety of tert-butyl alkyl peroxoates afforded the corresponding alkyl fluorides upon treatment with NFSI (Scheme 20) [71]. Primary alkyl fluoride formation was not efficient, which supports the mechanism hypothesis of radical intermediates.

The intermolecular amino fluorination of styrene's (Scheme 21) [72] can be facilitated by the use of palladium catalysts as described by Liu. Although reactions accomplish amino fluorination of alkenes, different approaches were employed and different reaction mechanisms proposed for each case. Intermolecular amino fluorination is thought to occur via fluoro- palladation involving substrate, NFSI and the active palladium complex, followed by oxidation to a putative Pd (IV) species, and subsequent reductive elimination to form a carbon-nitrogen bond. The formation C-F bonds at sp3 carbon centers by reductive elimination from high-valent transition-metal complexes has also been investigated with Pt (IV) complexes [73,74].
Enantiopure (R)-silyl ketones were prepared by diastereoselective silylation of the (S)-or (R)-1-amino-2- methoxy methylpyrrolidine (SAMP/RAMP) hydrazone and used as substrates in diastereoselective electrophilic fluorinations in which the silyl group acts as a traceless directing group. Lithium enolates of chiral compound generated by LDA were fluorinated with enantiopure (R)-silyl ketones were prepared by diastereoselective silylation of the (S)-or(R)-1-amino-2- methoxymethylpyrrolidine (SAMP/RAMP) hydrazone and used as substrates in diastereoselective electrophilic fluorinations in which the silyl group acts as a traceless directing group [75].
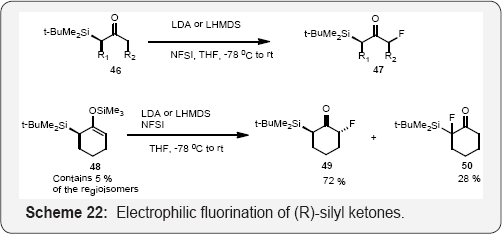
Lithium enolates of compound generated by LDA were fluorinated with NFSI in good yields and with high diastereomeric excesses. Interestingly, LiHMDS allowed reverse diastereoselectivity to be obtained. The diastereoselectivity was found to reflect the ratio of enolate stereomers, with NFSI reacting only from the less sterically hindered enolate face. This concept was also applied to silyl enol ether; however, the fluorination gave rise to a significant amount of regioisomers (Scheme 22) [76].
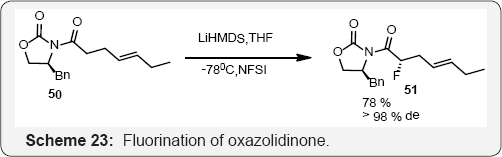
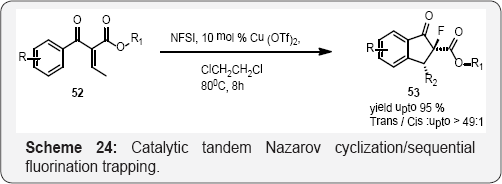
A chiral oxazolidinone auxiliary was also used by Stauton and co-workers to direct the addition of a fluorine atom in the preparation of fluoro analogue as a biosynthetic precursor of the ionophore antibiotic tetronasin (Scheme 23) [77]. A new catalytic stereo selective tandem transformation via Nazarov cyclization/ electrophilic fluorination has been accomplished (Scheme 24). This sequence is efficiently catalyzed by a Cu (II) complex to afford fluorine-containing 1-indanone derivatives with two new stereocenters with high diastereoselectivity (trans/cis up to 49/1). Three examples of catalytic enantioselective tandem transformation are presented [78] (Scheme 25).
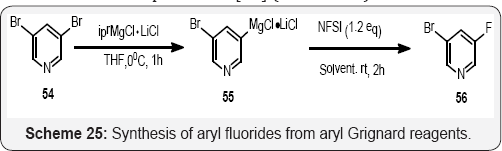
In (Scheme 25), Paul Knochel and co-workers developed a simple, convenient and highly versatile one-pot method for converting aromatic and heteroaromatic bromides or iodides into the corresponding fluorides by choosing an optimized different solvent mixture. This procedure allows a direct access to fluorinated pyridines, thiophenes, pyrroles and isoquinolines as well as to sterically congested fluorine-substituted benzenes, which are otherwise difficult to prepare. Further investigations of this potentially practical synthetic method are currently underway [79] (Scheme 26).
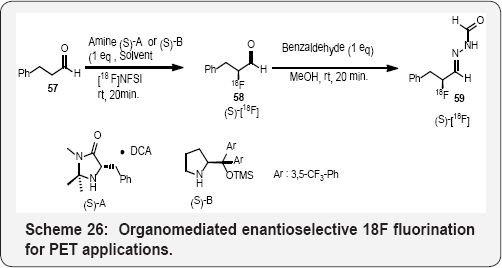
The first organomediated asymmetric 18F fluorination has been accomplished using a chiral imidazolidinone and [18F] N-fluorobenzenesulfonimide in (Scheme 26). This method provides access to enantioenriched18F-labeled a-fluoroaldehydes (>90% ee), which are versatile chiral 18F synthons for the synthesis of radiotracers. The utility of this process is demonstrated with the synthesis of the PET (positron emission tomography) tracer (2S,4S)-4-[18F] fluoroglutamic acid [80](Scheme 27).
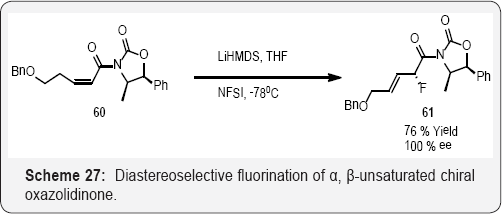
Diastereoselective fluorination of α,β-unsaturated chiral oxazolidinone was conducted by reaction of LiHMDS followed by addition of NFSI to produce a single diastereomer in 76% yield (Scheme 27).The complete diastereoselectivity reached with NFSI, compared to 82% de with NFOBS, was attributed to the greater steric bulk of NFSI. The reaction provided a nice example of deconjugative electrophilic fluorination. The acyclic fluoro compound was employed in the synthesis of fluoro carbohydrates [81,82] (Scheme 28). Baoming and co workers reports in (Scheme 28) that a convenient and efficient method for fluorination of methylene cyclopropanes is reported. This is exemplified in the stereo selective preparation of N-[(E)-3- fluorobut-3-en-1-yl]-benzene sulfonimides by the reaction of methylene cyclopropanes with N-fluorobenzene sulfonimide (NFSI) in good to excellent yields [83].
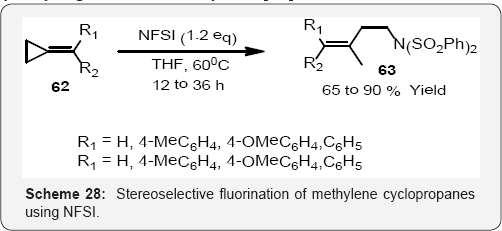
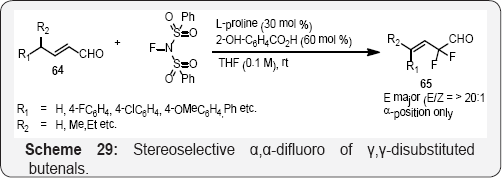
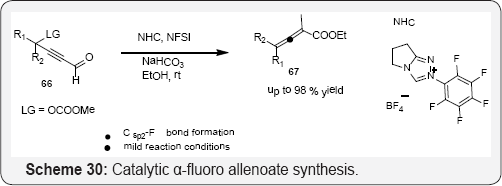
The allylic moieties has been found in many bioactive compounds and medicines, and compounds with a fluorine atom at the α-position of an allylic moiety, allylic fluorides, have exhibited excellent enhancement of the bioactivity of their parent compounds [84,85]. Furthermore, allyl fluorides have served as versatile intermediates in the synthesis of a large number of fluorinated compounds [86,87] motivating the development of numerous synthetic methods with controlled -regio and stereoselectivity for allylic fluorides in (scheme 29) and (Scheme 30) [88,89].
The first catalytic a-fluoro allenoate synthesis is described by complete the structure with a suitable combination of N-heterocyclic carbine precatalyst, base, and fluorine reagent, the reaction proceeded smoothly to yield a wide range of α-fluoro allenoates with excellent chemoselectivity (Scheme 30). These substituted a-fluorinated allenoates have been synthesized for the first time, and they are versatile synthetic intermediates toward other useful fluorine-containing building blocks [90].
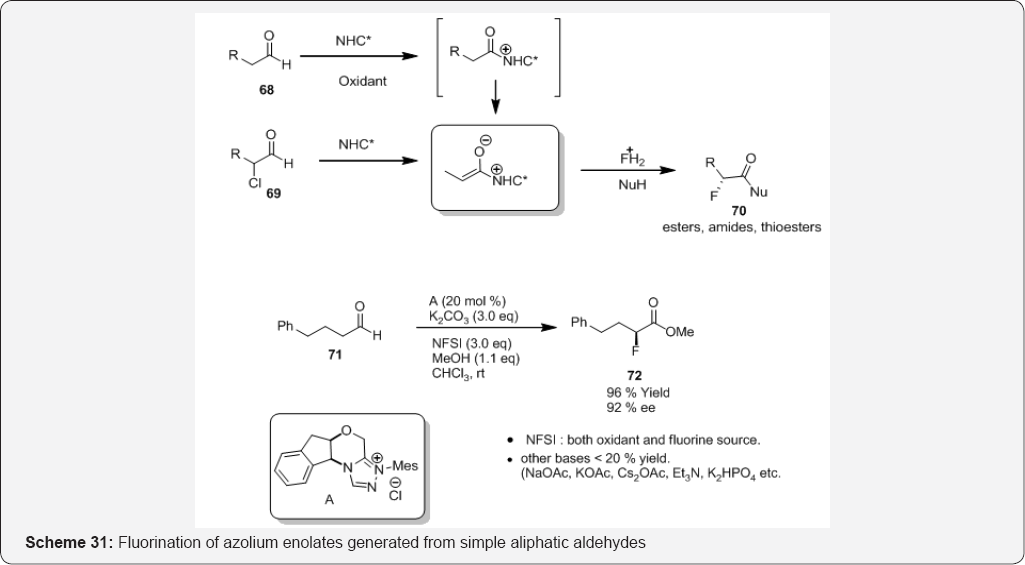
The asymmetric fluorination of azolium enolates that are generated from readily available simple aliphatic aldehydes or α-chloro aldehydes and N-heterocyclic carbenes (NHCs) is described in (Scheme 31). The process significantly expands the synthetic utility of NHC-catalyzed fluorination and provides facile access to a wide range of α-fluoro esters, amides, and thioesters with excellent enantioselectivity [91]. Catalytic systems that are based on N-heterocyclic carbenes (NHCs) have proven to be versatile in particular for establishing the α-stereogenic center of esters via key azolium enolate intermediates [92](Scheme 32).
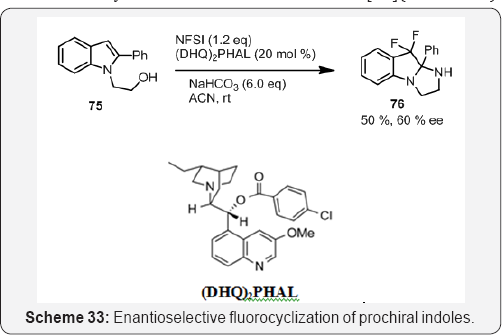
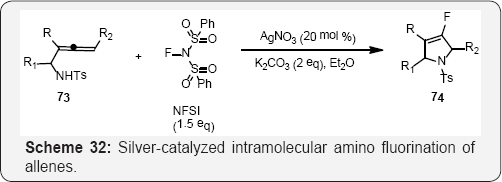
Guosheng Liu and co workers reported a novel silver- catalyzed intramolecular amino fluorination of allenes for the synthesis of 4-fluoro-2,5-dihydropyrroles, in which the vinyl C-Ag bond is cleaved using NFSI to afford vinyl C-F bonds [93]. In addition, further convenient aromatization of fluorinated dihydropyrroles readily afforded the corresponding 4-fluoropyrrole derivatives (Scheme 33). In Scheme 33, Prochiral indole 75 was also subjected to the optimized fluorocyclization conditions, which afforded the difluorinated tricyclic tetrahydro oxazolo [3,2-a] indole 76 in 50% yield, 60% ee [94] (Scheme 34).
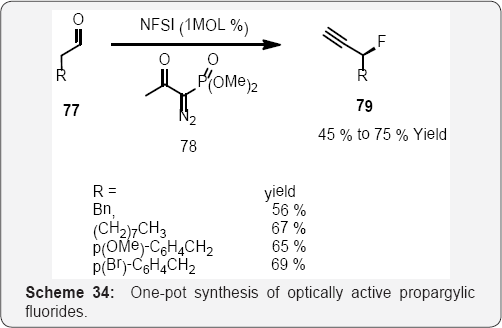
In (Scheme 34), Jθrgensen and co-workers reported a simple, direct one-pot organocatalytic approach to the formation of optically active propargylic fluorides 79. This consists of organocatalytic α-fluorination of aldehydes followed by homologation with the Ohira-Bestman reagent 78, providing optically active propargylic fluorides [95,96].
Conclusion
The most significant, conceptual advances over the past decade in the area of fluorination, broadly defined, were made in the reactions that led to the formation of C-F, most prominently by organo and transition-metal catalysis. The most challenging transformation remains the formation of the parent C-F bond, primarily due to the high hydration energy of fluoride, strong metal-fluorine bonds and the highly polarized nature of bonds to fluorine. Reagents of the NF class, several of which are now commercially available, provide the organic chemist with a relatively safe and practical means of selectively positioning fluorine at chosen carbanionic-type sites in molecules. However, the reagents stability in storage and ease of use are achieved at the cost of employing an R2N or R3N+-organic carrier. For many large-scale uses elemental fluorine, somehow “tamed” to act as a predictably selective electrophile, would ultimately be the most economical and environmentally “greener” alternative.
References
- F Swarts, AR Bull (1898) Modern Synthesis Processes and Reactivity of Fluorinated Compounds. Med Belg 35: 375-420.
- HJ Bchm, D Banner, S Bendels, M Kansy, B Kuhn, et al. (2004) Fluorine in Medicinal Chemistry. ChemBioChem 5: 637-643.
- JP Begue, DB Delphon (2006) Recent Advances (1995-2005) in Fluorinated Pharmaceuticals based on Natural Products. J Fluorine Chem 127: 992-1012.
- KL Kirk (2006) Fluorine in Medicinal Chemistry: Recent Therapeutic Applications of Fluorinated Small Molecules. J Fluorine Chem 127: 1013-1029.
- K Muller, C Faeh, F Diederich (2007) Science 317: 1881-1886.
- WK Hagmann (2008) The Many Roles for Fluorine in Medicinal Chemistry. J Med Chem 51: 4359-4369.
- S Purser, PR Moore, S Swallow, V Gouverneur (2008) Fluorine in Medicinal Chemistry. Chem Soc Rev 37: 320-330.
- P Jeschke (2004) The Unique Role of Fluorine in the Design of Active Ingredients for Modern Crop Protection. ChemBioChem 5(5): 570589.
- M Pagliaro, R Ciriminna (2005) New Fluorinated Functional Materials. J Mater Chem 15: 4981-4991.
- C Hansch, A Leo (1979) Substituent Constants for Correlation Analysis in Chemistry and Biology. John Wiley & Sons Inc, USA. p. 352.
- JA Wilkinson (1992) Recent Advances in the Selective Formation of the Carbon-Fluorine Bond. Chem Rev 92(4): 505-519.
- J Wang, MS-Rosello, JL Acena, C del Pozo, AE Sorochinsky, et al. (2014) Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001-2011). Chem Rev 114: 2432-2506.
- LS German, SV Zemskov (1989) New Fluorinating Agents in Organic Synthesis. Springer-Verlag Berlin Heidelberg Berlin IX p. 283.
- S Rozen (1988) Elemental Fluorine as a Legitimate Reagent for Selective Fluorination of Organic Compounds. Acc Chem Res 21(8): 307-312.
- S Rozen (1996) Elemental Fluorine: Not Only for Fluoroorganic Chemistry. Acc Chem Res pp. 29(5): 243-248.
- SD Taylor, CC Kotoris, G Hum (1999) Recent Advances in Electrophilic Fluorination. Tetrahedron 55: 12431-12477.
- RD Chambers (2004) Fluorine in Organic Chemistry. Blackwell Publishing Ltd pp. 1-406.
- M Hudlicky, AE Pavlath (1995) Chemistry of Organic Fluorine Compounds II: A Critical Review. ACS Monograph Series 187 Washington, DC: American Chemical Society.
- S Rozen (1996) Selective Fluorinations by Reagents Containing the OF Group. Chem Rev 96: 1717-1736.
- G Villalba, RU Ayres, H Schroder (2007) Accounding for Fluorine: Production, Use, and Loss. J Ind Ecol 11: 85-101.
- WA Sheppard (1969) Mechanism of Fluorination by Perchloryl Fluoride. Tetrahedron Lett 10: 83-84.
- MA Tius (1995) Xenon Difluoride in Synthesis. Tetrahedron 51: 6605-6634.
- E Differding, H Ofner (1991) N-Fluorobenzenesulfonimide: A Practical Reagent for Electrophilic Fluorination. Synlett 1991: 187189.
- WE Barnette (1984) N-Fluoro-N-alkylsulfonamides: Useful Reagents for the Fluorination of Carbanions. J Am Chem Soc 106(2): 452-454.
- T Umemoto, K Kawada, K Tomita (1986) N-Fluoropyridinium Triflate and its Derivatives: Useful Fluorinating Agents. Tetrahedron Lett 27: 4465-4468.
- RE Banks, SN Mohialdinkhaffaf, GS Lal, I Sharif, RG Syvret, et al. (1992) l-Alkyl-4-fluoro-l,4-diazoniabicyclo[2.2.2]octane Salts : A Novel Family of Electrophilic Fluorinating Agents. J Chem Soc Chem Commun pp. 595-596.
- TN Paul, SG Durn, MD Burkart, SP Vincent, CH Wong, et al. (2005) Selectfluor: Mechanistic Insight and Applications. Angew Chem Int Ed 44(2): 192-212.
- T Umemoto, K Kawada, K Tomita (1986) N-Fluoropyridinium Triflate and its Derivatives: Useful fluorinating Agents. Tetrahedron Lett 27: 4465-4468.
- T Umemoto, S Fukami, G Tomizawa, K Harasawa, K Kawada, et al. (1990) Power and Structure-Variable Fluorinating Agents: The N-Fluoropyridinium Salt System. J Am Chem Soc 112(23): 85638575.
- G Resnati, DD DesMarteau (1991) N-Fluorobis[ (trifluoromethyl) sulfonyl]imide: An Efficient Reagent for the a-Fluorination of Functionalized Carbonyl Compounds. J Org Chem 56 (16): 49254929.
- DD DesMarteau, ZQ Xu, M Witz (1992) N-Fluorobis[ (perfluoroalkyl) sulfonyl]imides: Reactions with Some Olefins via a-Fluoro Carbocationic Intermediates. J Org Chem 57(2): 629-635.
- M Zupan, S Stavber, SP Tjasii, P Maja (1996) Ritter-type Fluorofunctionalisation as a New, Effective Method for Conversion of Alkenes to Vicinal Fluoroamides. Chem Commun: 2247-2248.
- RE Banks (1998) Selectfluor™ Reagent F-TEDA-BF4 in Action: Tamed Fluorine at Your Service. J Fluorine Chem 87: 1-17.
- RE Banks, MK Besheesh, SNM Khaffaf, I Sharif (1996) N-Halogeno compounds Part l8. l-Alky1-4-Fluoro-l,4-diazoniabicyclo [2.2.21 Octane Salts: User Friend1y Site Selective Electrophilic Fluorinating Agents of the N-Fluoroammonium Class. J Chem Soc Perkin Trans 1: 2069-2076.
- GS Lal, GP Pez, RG Syvret (1996) Electrophilic NF Fluorinating Agents. Chem Rev 96(5):1737-1756.
- P Anbarasan, H Neumann, M Beller (2010) Efficient Synthesis of Aryl Fluorides. Angew Chem Int Ed 49: 2219-2222.
- T Umemoto, S Fukami, G Tomizawa, K Harasawa, K Kawada, et al. (1990) Power and Structure-Variable Fluorinating Agents. The N-Fluoropyridinium Salt System. J Am Chem Soc 112(23) : 85638575.
- JA Ma, D Cahard (2004) Screening of Chiral Catalysts for Enantioselective Electrophilic Fluorination of p-Ketoesters. J Fluorine Chem 125: 1357-1361.
- KL Hull, WQ Anani, MS Sanford (2006) Palladium-Catalyzed Fluorination of Carbon-Hydrogen Bonds. J Am Chem Soc 128(22): 7134-7135.
- AR Dick, KL Hull, MS Sanford (2004) A Highly Selective Catalytic Method for the Oxidative Functionalization of C-H Bonds. J Am Chem Soc 126(8): 2300-2301.
- LV Desai, KL Hull, MS Sanford (2004) Palladium-Catalyzed Oxygenation of Unactivated sp3 C-H Bonds. J Am Chem Soc 126(31): 9542-9543.
- D Kalyani, NR Deprez, LV Desai, MS Sanford (2005) Oxidative C-H Activation/C-C Bond Forming Reactions: Synthetic Scope and Mechanistic Insights. J Am Che Soc 127(20): 7330-7331.
- JC Lewis, JY Wu, RG Bergman, JA Ellman (2005) Microwave-Promoted Rhodium-Catalyzed Arylation of Heterocycles through C-H Bond Activation. Angew Chem Int Ed 45: 1589-1591.
- H Sun, Y Zhang, P Chen, YD Wu, X Zhang, et al. (2016) Ligand-Assisted Palladium(II)/(IV)Oxidation for sp3 C-H Fluorination. Adv Synth Catal 358: 1946-1957.
- Y Hamashima, K Yagi, H Takano, L Tamas, M Sodeoka, et al. (2002) An Efficient Enantioselective Fluorination of Various p-Ketoesters Catalyzed by Chiral Palladium Complexes. J Am Chem Soc 124(49): 14530-14531.
- C Baudequin, JC Plaquevent, C Audouard, D Cahard (2002) Enantioselective Electrophilic Fluorination in Ionic Liquids. Green Chem 4: 584-586.
- N Shibata, T Ishimaru, E Suzuki, KL Kirk (2003) Enantioselective Fluorination Mediated by N-Fluoroammonium Salts of Cinchona Alkaloids: First Enantioselective Synthesis of BMS-204352 (MaxiPost). J Org Chem 68(6): 2494-2497.
- JA Ma, D Cahard (2004) Copper(II) Triflate-bis(oxazoline)-Catalysed Enantioselective Electrophilic Fluorination of p-Ketoesters. Tetrahedron: Asymmetry 15: 1007-1011.
- E Cazorla, B Metay, M Andrioletti (2009) Metal-free electrophilic fluorination of alkyl trifluoroborates and boronic acids. Tetrahedron Lett 50: 3936-3938.
- DW Slocum, P Shelton, KM Moran (2005) Processing Aryllithium and Hetaryllithium Intermediates: Formation of Halogen and Chalcogen Derivatives. Synthesis 20: 3477-3498.
- P Anbarasan, H Neumann, M Beller (2010) A new and practical Grignard-coupling-fluorination sequence: synthesis of 2-aryl- fluoroarenes. Chem Asian J 5: 1775-1777.
- P Anbarasan, H Neumann, M Beller (2010) Efficient Synthesis of Aryl Fluorides. Angew Chem Int Ed 49: 2219-2222.
- S Yamada, A Gavryushin, P Knochel (2010) Convenient Electrophilic Fluorination of Functionalized Aryl and Heteroaryl Magnesium Reagents. Angew Chem Int Ed 49: 2215-2218.
- H Daniel, MT Paull, AD Ethan, R Leland, WT Lectka, et al. (2008) Catalytic, Asymmetric a-Fluorination of Acid Chlorides: Dual Metal- Ketene Enolate Activation. J Am Chem Soc 130(51): 17260-17261.
- N Shibata, J Kohno, K Takai, T Ishimaru, S Nakamura, et al. (2005) Highly Enantioselective Catalytic Fluorination and Chlorination Reactions of Carbonyl Compounds Capable of Two-Point Binding. Angew Chem Int Ed 44: 4204-4207.
- M Althaus, A Togni, A Mezzetti (2009) Asymmetric Oxidative a-Fluorination of 2-Alkyl Phenyl Acetaldehydes with AgHF2 and Ruthenium/PNNP Catalysts. J Fluorine Chem 130: 702-707. 2
- S Suzuki, H Furuno, Y Yokoyama, J Inanaga (2006) Asymmetric Fluorination of p-Keto Esters Catalyzed by Chiral Rare Earth Perfluorinated Organophosphates. Tetrahedron: Asymmetry 17(4): 504-507.
- S Suzuki, Y Kitamura, S Lectard, Y Hamashima, M Sodeoka, et al. (2012) Catalytic Asymmetric Mono-Fluorination of a-Keto Esters: Synthesis of Optically Active p-Fluoro-a-Hydroxy and p-Fluoro-a- Amino Acid Derivatives. Angew Chem In Ed 51: 4581-4585.
- DS Reddy, N Shibata, J Nagai, S Nakamura, T Toru, et al. (2008) Desymmetrization-like Catalytic Enantioselective Fluorination of Malonates and its Application to Pharmaceutically Attractive Molecules. Angew Chem Int Ed 47: 164-168.
- D Teresa, D Beeson, WC MacMillan (2005) Enantioselective Organocatalytic α-Fluorination of Aldehydes. J Am Chem Soc 127(24): 8826-8828.
- Y Hamashima, K Yagi, H Takano, L Tamas, M Sodeoka, et al. (2002) An Efficient Enantioselective Fluorination of Various β-Ketoesters Catalyzed by Chiral Palladium Complexes. J Am Chem Soc 124(49): 14530-14531.
- Y Hamashima, T Suzuki, H Takano, Y Shimura, Y Tsuchiya, et al. (2006) Highly Enantioselective Fluorination Reactions of β-Ketoesters and β-Ketophosphonates Catalyzed by Chiral Palladium Complexes. Tetrahedron 62(30): 7168-7179.
- Y Hamashima, T Suzuki, H Takano, Y Shimura, M Sodeoka, et al. (2005) Catalytic Enantioselective Fluorination of Oxindoles. J Am Chem Soc 127(29): 10164-10165.
- T Suzuki, T Goto, Y Hamashima, M Sodeoka (2007) Enantioselective Fluorination of tert-Butoxycarbonyl Lactones and Lactams Catalyzed by Chiral Pd(II)-Bisphosphine Complexes. J Org Chem 72(1): 246250.
- D Enders, MRM Huttl (2005) Direct Organocatalytic a-Fluorination of Aldehydes and Ketones. Synlett 6: 0991-0993.
- TD Beeson, DWC MacMillan (2005) Enantioselective Organocatalytic α-Fluorination of Aldehydes. J Am Chem Soc 127(24): 8826-8828.
- M Marigo, D Fielenbach, A Braunton, A Kjoersgaard, KA Jorgensen, et al. (2005) Enantioselective Formation of Stereogenic Carbon- Fluorine Centers by a Simple Catalytic Method. Angew Chem Int Ed 44: 3703-3706.
- DD Steiner, N Mase, CF Barbas (2005) Direct Asymmetric α-Fluorination of Aldehydes. Angew Chem Int Ed 44(24): 3706-3710.
- P Kwiatkowski, TD Beeson, JC Conrad, DWC MacMillan (2011) Enantioselective Organocatalytic α-Fluorination of Cyclic Ketones. J Am Chem Soc 133(6): 1738-1741.
- ML Schulte, CW Lindsley (2011) Highly Diastereoselective and General Synthesis of Primary β-Fluoroamines. Org Lett 13(20): 56845687.
- CP Andrieux, E Differding, M Robert, JM Saveant (1993) Controlling Factors of Stepwise versus Concerted Reductive Cleavages. Illustrative Examples in the Electrochemical Reductive Breaking of Nitrogen-Halogen Bonds in Aromatic N-Halosultams. J Am Chem Soc 115(15): 6592-6599.
- MR Becerril, CC Sazepin, JCT Leung, T Okbinoglu, P Kennepohl, et al. (2012) Fluorine Transfer to Alkyl Radicals. J Am Chem Soc 134(9): 4026-4029.
- S Qiu, T Xu, J Zhou, Y Guo, G Liu, et al. (2010) Palladium-Catalyzed Intermolecular Aminofluorination of Styrenes. J Am Chem Soc 132(9): 2856-2857.
- NP Mankad, FD Toste (2012) C (sp3)-F Reductive Elimination from Alkyl Gold (III) Fluoride Complexes. Chem Sci 3(1): 72-76.
- T Wu, G Yin, G Liu (2009) Palladium-Catalyzed Intramolecular Aminofluorination of Unactivated Alkenes. J Am Chem Soc 131(45): 16354-16355.
- D Enders, M Potthoff, G Raabe, J Runsink (1997) Regio and Enantioselective Synthesis of α-Fluoroketones by Electrophilic Fluorination of a-Silylketone Enolates with N-Fluorobenzosulfonimide. Angew Chem Int Ed Engl 36: 23622364.
- D Enders, S Faure, M Potthoff, J Runsink (2001) Diastereoselective Electrophilic Fluorination of Enantiopure α-Silylketones using N-Fluorobenzosulfonimide: Regio and Enantioselective Synthesis of α -Fluoroketones. Synthesis pp. 2307-2319.
- SL Less, S Handa, K Millburn, PF Leadlay, CJ Dutton, et al. (1996) Biosynthesis of Tetronasin: Part 6. Preparation of Structural Analogues of the Diketide and Triketide Biosynthetic Precursors to Tetronasin. Tetrahedron Lett 37(20): 3515-3518.
- J Nie, HW Zhu, HF Cui, MQ Hua, JA Ma, et al. (1997) Catalytic Stereoselective Synthesis of Highly Substituted Indanones via Tandem Nazarov Cyclization and Electrophilic Fluorination Trapping. Org Lett 9(6): 3053-3056.
- S Yamada, A Gavryushin, K Paul (2010) Convenient Electrophilic Fluorination of Functionalized Aryl and Heteroaryl Magnesium Reagents. Angew Chem Int Ed 49: 2215-2218.
- F Buckingham, AK Kirjavainen, S Forsback, A Krzyczmonik, T Keller, et al. (2015) Organomediated Enantioselective 18FFluorination for PET Applications. Angew Chem Int Ed 54: 13366-13369.
- FA Davis, H Qi (1996) Asymmetric Synthesis of 2-Deoxy-2-Fluoro-y- Aldonolactones and their Conversion to 2-Deoxy-2- Fluoropentoses. Tetrahedron Lett 37: 4345-4348.
- FA Davis, H Qi, G Sundarababu (2000) Application of Oxazolidinone a-Fluoro Amide Chiral Building Blocks in the Asymmetric Synthesis of Fluorinated Carbohydrates: 2-Deoxy-2-fluoropentoses. Tetrahedron 56(30): 5303-5310.
- W Fu, G Zou, BM Zhu, D Hong, D Deng, et al. (2009) Stereoselective Fluorination of Methylenecyclopropanes with N-F reagents: A modular Entry to γ-Fluorohomoallylic Sulfonimides and γ-Fluorohomoallylic Amides. Journal of Fluorine Chemistry 130(11): 996-1000.
- M Tredwell, V Gouverneur (2006) Electrophilic Fluorination of Organosilanes. Org Biomol Chem 4: 26-32.
- MO Khan, H Lee (2008) Synthesis and Pharmacology of AntiInflammatory Steroidal Antedrugs. Chem Rev 108(12): 5131-5145.
- LE Combettes, M Schuler, R Patel, B Bonillo, B Odell, et al. (2012) Synthesis of 3-Fluoropyrrolidines and 4-Fluoropyrrolidin-2-ones from Allylic Fluorides. Chem Eur J 18: 13126-13132.
- JAB Laurenson, S Meiries, JM Percy, R Roig (2009) Allylic fluorination via an unusual alkene Z/E isomerisation. Tetrahedron Lett. 50(26): 3571-3573.
- J Wu (2014) Review of Recent Advances in Nucleophilic C-F Bond -Forming Reactions at sp3 Centers. Tetrahedron Lett 55: 4289-4294.
- C Hollingworth, V Gouverneur (2012) Transition Metal Catalysis and Nucleophilic Fluorination. Chem Commun 48: 2929-2942.
- W Xu, Z Wu, J Wang (2016) a-Fluoroallenoate Synthesis via N-Heterocyclic Carbene-Catalyzed Fluorination Reaction of Alkynals. Org Lett 18(3): 576-579.
- X Dong, W Yang, W Hu, J Sun (2015) N-Heterocyclic Carbene Catalyzed Enantioselective α-Fluorination of Aliphatic Aldehydes and α-Chloro Aldehydes: Synthesis of α-Fluoro Esters, Amides, and Thioesters. J Angew Chem Int Ed 54: 660-663.
- HU Vora, P Heeler, T Rovis (2012) Exploiting Acyl and Enol Azolium Intermediates via N-Hetero- cyclic Carbene-Catalyzed Reactions of a-Reducible Aldehydes. Adv Synth Catal 354: 1617-1639.
- X Tao, L Guosheng (2012) Silver Catalysed Fluorination Reactions. Synlett 23(7): 955-958.
- O Lozano, G Blessley, T Martinez, L Amber, GT Thompson, et al. (2011) Organocatalyzed Enantioselective Fluorocyclizations. Angew Chem Int Ed 50: 8105-8109.
- H Jiang, A Falcicchio, KL Jensen, MW Paixao, S Bertelsen, et al. (2009) Target-Directed Organocatalysis: A Direct Asymmetric Catalytic Approach to Chiral Propargylic and Allylic Fluorides. J Am Chem Soc 131(20): 7153-7157.






























