Anti-Diarrhoeal Properties of Cis-9-Octadecenoic Acid Isolated From Landolphia Owariensis Plant
*S Garba1and I Garba2
1Chemistry Department Nigerian Defence Academy, Africa
2NNPC-Krpc Kaduna, Africa
Submission: September 01, 2017; Published: September 18, 2017
*Corresponding author: S Garba, Chemistry Department Nigerian Defence Academy, Nigeria, Africa, Email: sgarba@nda.edu.ng
How to cite this article: S Garba, Garba. Anti-Diarrhoeal Properties of Cis-9-Octadecenoic Acid Isolated From Landolphia Owariensis Plant. Organic & Medicinal Chem IJ. 2017; 3(4): 555619. DOI: 10.19080/OMCIJ.2017.03.555619.
Abstract
The phytochemical screening and the antidiarrhoeal activities of the crude extracts from the leaves Landolphiaowariensis were carried out using standard methods. The results of the phytochemical screening showed that the plant contained alkaloids, saponins, tannins, flavonoids and glycosides. The results of the antidiarrhoeal screening of the crude extracts showed that Landolphiaowariensis exhibited zone of inhibition ranging from 15 mm to 35 mm against test microbes. Ethyl-acetate extract of the Landolphiawariensis being the most active fraction was subjected to column chromatography, leading to the isolation of single compound that showed a high activities (zone of inhibition from 8mm to 32mm) against these causative agents of diarrhoea (Salmonella Typhi, Shigella dysentriae and Escherichia Coli respectively). Spectroscopic analysis of the isolated compound using JHNMR, 13CNMR, IR and GCMS showed that the compound was Cis-9-Octadecenoic acid. The result of this works therefore showed that the extract from the leaves of Landolphiaowariensis could be used for the treatment of diarrhoea and other diseases caused by targeted microorganisms.
Keywords: Antidiarrheal; Landolphiaowariensis; Isolation; Cis-9-Octadecenoic Acid
Introduction
Plants are important in our everyday existence. They provide our foods, produce the oxygen we breathe, and serve as raw materials for many industrial products such as clothes, foot wears and also provide raw materials for our buildings and in the production of bio-fuels, dyes, perfumes, pesticides, adsorbents and drugsetc. Rahmatullah et al. [1].
Medicinal plants have been used in healthcare since time immemorial. Studies have been carried out globally to verify their efficacy and some of the findings have led to the production of plant-based medicines Sofowora et al. [2].
Since the advent of modern allopathic medicine, over 90% of traditional medicine recipes remedies contain medicinal plants Sofowora et al. [2] the use of traditional medicine (inclusive of the use of medicinal plants for cure) declined to a considerable extent. However, in recent years, traditional medicine has made a comeback for a variety of reasons including side-effects and toxicity of modern synthetic drugs, evolution of multi-drug resistance microorganisms, and the inability of modern medicine to find effective cures for a number of diseases. Landolphiaowariensis (Family Apocynaceae) is one of these plants found in the savannah forest, about 100m in height and 1m in girth. It can be found in west Cameroon, Guinea, Sudan, Uganda, Southern Tanganyika and West Africa region. Of all species of Landolphia, Landolphiaowariensis is the commonest; it is commonly called vine rubber and known locally by various names in Nigeria: Ibo-Eso/Utu, Yoruba-Mba and Hausa-Ciwo Galadima et al.[3].
Diarrhoea is an etiologically diverse condition unlike some other infectious diseases such as tuberculosis, HIV/AIDS and malaria since it is caused by a variety of enteric pathogens including bacteria, viruses and protozoa Tannaz et al. [4].
Oral rehydration therapy has been the key strategy for effective management of diarrhoea but was met with little success. There are also attempt in the past to develop vaccines against diarrhoea causing organisms that receives little or no encouraging responses in developing countries Tannazet al. [4]. Hence, the focus on medicinal plants which was widely believe to be a source of developing cost effective alternative approaches for treatment of diarrhea. Medicinal plants have recently gained popularity as prospective anti-diarrheal agents as can be judged by the number of studies that have been taken to search for alternative cure for diarrhea. The research is aimed at assessing the anti-diarrhea properties of Landolphiaowariensis with a view of isolating and characterizes the most active component of the plant that can be used for the treatment of diarrhea.
Experimental
Sample Collection
Ariel part of the plants were collected from Doka area of Kachia Local Government area of Kaduna state, and identified by Mallam Musa at the Herbarium of Biological Sciences Department, Faculty of Science, Ahmadu Bello University Zaria, Nigeria. A voucher specimen with a number 901442 was deposited in the herbarium.
Extraction of the Sample
A portion (200g) of powdered Landolphiaowariensis was percolated into 450 cm3 of methanol for two weeks, and then filtered. The filtrate was evaporated at 400C using rotary evaporator and the extract allowed to dry and weighed. The crude extract of Landolphiaowariensis was then dissolved in 10cm3 of distilled water and transferred into a separator funnel. Equal volume of petroleum-ether was added, and the resulting mixture was shaken thoroughly, and allowed to stand overnight for the two layers to separate. The two layers were separately drained off, and the procedure was repeated until all the petroleum ether soluble fraction were completely remove, and finally the two layers were evaporated separately using rotary evaporator, the same procedure was repeated using ethyl-acetate and methanol, three crude extracts were obtained at the end of the extraction processes (petroleum-ether, ethyl-acetate and methanol crude extracts respectively).
Phytochemical Analysis
The phytochemical analyses of the crude extracts were carried out using standard methods Sofowora [2].
Antimicrobial Analysis
The antimicrobial screening of the crude extracts was carried out using agar well diffusion method described by sofowora, 1993 Trease and Evans [5]. The most potent extract was subjected to chromatographic analyses leading to the isolation of a one compound having antidrrheal property against the teat microbes.
Results and Discussion
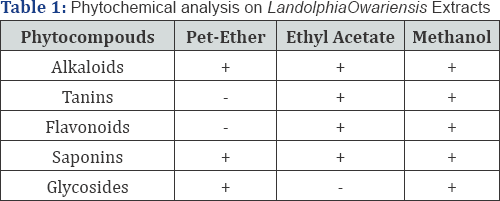
The result of the phytochemical screening of the petroleum ether, ethyl acetate and methanol extracts of Landolphiaowariensis (Table 1) showed that they contains alkaloids, tannins, flavonoids, saponins, and glycosides. Similar observation where made on the plants extracts by Nwaogu et al. [6] and El-Mahmood et al. These phyto-compounds are known to be effective against diarrhoea caused by trypanosome and plasmodium, and possess the ability to initiate human physiological activities such as the stimulation of phagocytic cells and mediation of tumor activities Garba and Salihu [7-10] (Table 1).
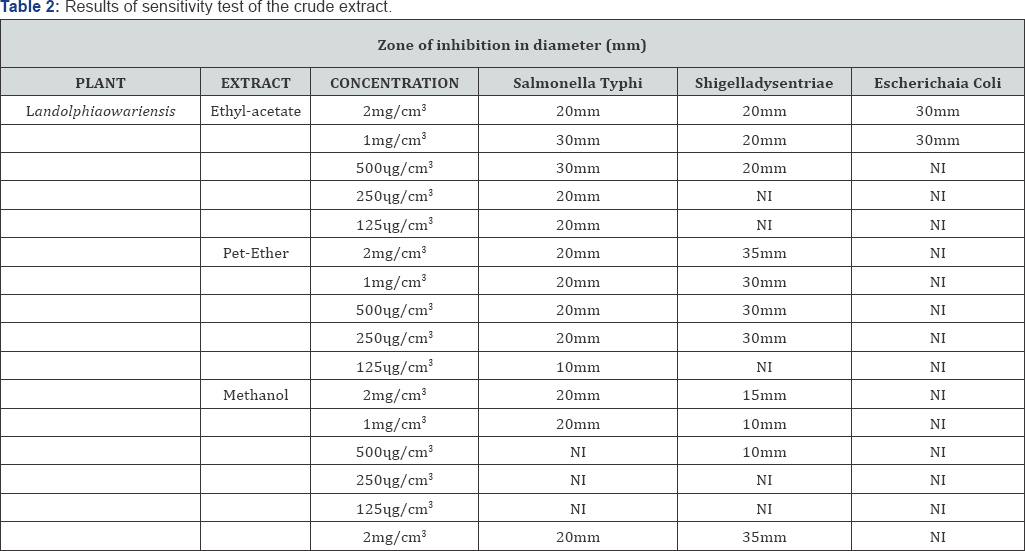
The result of the antimicrobial screening of the extracts (Table 2) showed that Landolphiaowariensis exhibited the highest activity, with zone of inhibition diameter of 35 mm for the petroleum ether extract against Shigella dysentriae, and 20 mm for salmonella typhi, while it showed no inhibition against Escherichia coli. The result also showed that ethyl acetate extract of Landolphiaowariensis showed a wide range of inhibition against the tested organism [11-13], having highest zone of inhibitions diameter of 30 mm, 20 mm, and 30 mm against salmonella typhi, Shigella dysentriae and Escherichia coli. This observed antimicrobial property is due to the presence of alkaloid, flavonoids, and tannin Nwaogu et al. [6] (Table 2).
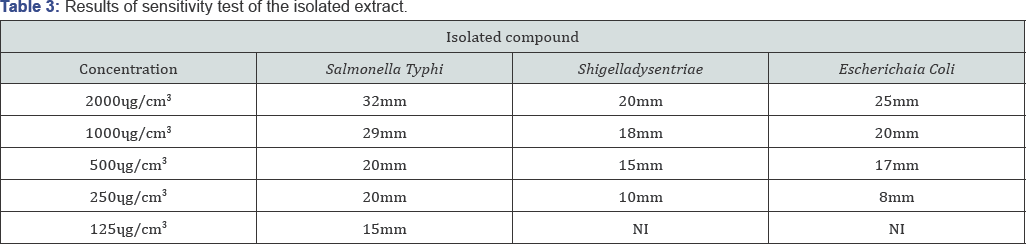
The result of the antimicrobial testof the isolated compound (Table 3) showed that the compound recorded a moderate to high antimicrobial properties against the bacteria isolates [14-17].
At 2000чg/cm3, the compound exhibited zone of inhibition of 32 mm, 20 mm, and 2 5 mm against Salmonella Typhi, Shigelladysentriae and Escherichaia Colirespectively. At 1000чg/ cm3, the compound exhibited activities (zone of inhibition diameter of 29 mm, 18 mm and 20 mm respectively) against Salmonella Typhi, Shigelladysentriae and Escherichaia Coli. At 500чg/cm3, the compound generally exhibited activities (zone of inhibition diameter of 20 mm, 15 mm and 17 mm respectively) against Salmonella Typhi, Shigelladysentriae and Escherichaia Coli [18-22]. At 250чg/cm3, the compound exhibited activities (zone of inhibition diameter of 20, 10 and 8 mm respectively) against Salmonella Typhi, Shigelladysentriae and Escherichaia Coli.
At 125чg/cm3, the compound exhibited activity (zone of inhibition of diameter 15 mm) against Salmonella Typhi, while it is inactive against Shigelladysentriae and Escherichaia Coli at that concentration (Table 3). Various types of fatty acids derivatives has been studied by a number of investigators using different technique and organisms, this research work further confirms the findings of Dilika et al, (2000) that oleic acid was active against staphylococcus aureus and Micrococcus Kristine. Furthermore, Arthur et al. [23,24] (1961) investigated the antimicrobial activity of oleic acid and find out that oleic acid has antimicrobial activity against some micro-organisms (Micrococcus pyogenes, penecilliumspp, Escherichiacoli, candidastellatoidea etc.) (Table 3).
Column Chromatography of the Crude Extracts:
A total of seventy five fractions were collected from the column chromatography, using petroleum ether: ethyl acetate (2:1). The Thin Layer Chromatography (TLC) of the fractions indicates that some are similar. Similar fractions were therefore pooled and recorded respectively (see Table 4) [25-27]. Fractions F2, F3, F4, F6, F7, F8, F9, F10, F12, F13, F14, F17, F18, F20, F21, F22, F23, F24, F25, F26, F29, 30, F31, F32, F33, F63 were pooled together as they have similar RF values (0.5) and coded E1, fractions F11, F15, F16, F35 were also pooled together as they have similar RF value (0.6) coded D1, fractions F19 and F28 were also pooled together as they have similar RF value (0.4) and coded C1, fraction F61 was collected separately and has RF value of 0.3 and coded B2, while fraction F5 was also collected separately and has RF value of 0.8 and coded A1.
Thin layer chromatography of fraction F5 was further carried out, and it gave a single spot of RF value of 0.8, indicating it is pure, as such; it was recrystallized to further purified the crystals and subjected to spectroscopic analysis. Table 4.6 showed the result of the column chromatography of ethyl acetate of Landolphiaowariensis pooled fractions and their weight [28-31]. The nature of the fraction ranges from yellowish through light brown to deep brown sticky solids (Figure 4).
Spectroscopic Analysis of the Isolated Compound
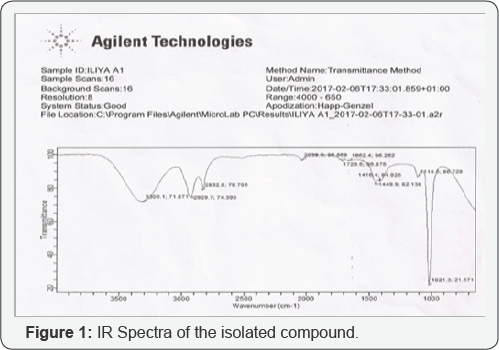
Infra-red spectroscopy (IR) of F5: The IR spectrum of compound F5 showed strong absorption at 3308.1 cm-1 which is due to OH band of carboxylic acid, 2929.7 cm'1 and 2832.8cm-1 are the various C-HSTR vibrational stretching of an alkane, 1416.4 is due to the presence methyl and methylene groups [32,33]. 1729.5 cm-1 is due to carbonyl stretching (C=O). The presence of methyl group was further confirmed by the presence of a peak at 1449.9 cm'1.
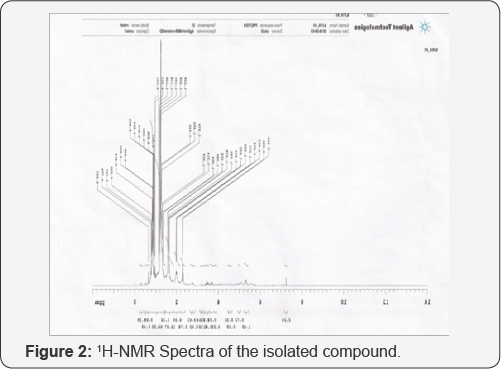
Nuclear Magnetic Spectroscopy (NMR) of F5: The 1HNMRand 13Cnmr (Figures 2 & 3) spectrum of compound F5 (Figure 2.0 and 3.0) was obtained by analyzing the sample on Agilent-NMR Technologies [34]. The 1HNMR spectrum (Figure 2.0) recorded signals at 7.3ppm being only single; it is assigned to the carboxylic (COOH) acid proton (H1), although 7.3ppm is absorption due to aromatic proton. A recorded signal at 5.32ppm is the methylene proton [H9 and H10]. A recorded signal at 1.558ppm are the various methylene protons [H3, H4, H5, H6, H7, H12, H13, H14, H15, and H16] in between CH=CH and COOH group and also in between CH=CH and CH3 group. A recorded signal 1.314ppm correspond to methylene proton [H17] attached to CH3 group. A recorded signal at 0.88ppm is the methyl proton [H18].
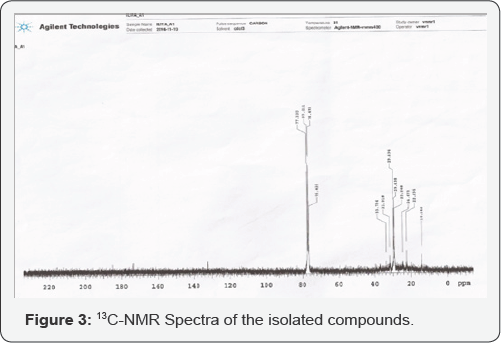
The 13Cnmr spectrum of compound F5 (Figure 2.0) had signal recorded at 14.14 ppm which corresponds to the methyl carbon (C18). Signal recorded at 22.7ppm is the methylene carbon (C17) attached to a methyl group. Signal recorded at 24.67ppm is the two methylene carbon (C16 and C4) attached to a methyleneclose to COOH group, and the other attached to a methylene close to CH3 group. Signal recorded at 29.308ppm is the methylene carbon (C15 and C5) attached in between COOH and CH2=CH2 groups and CH2=CH2 and CH3 groups. Signal recorded at 29.438ppm corresponds to the various methylene carbons [C6, C7, C12, C13, and C14] attached to CH2=CH2 group. Signal recorded at 29.7ppm are the two methylene carbon (C8, C11) attached CH2=CH2 group. Signal recorded at 31.918ppm is the methylene carbon (C3) attached to CH2 close to COOH group. Signal recorded at 33.918ppm corresponds to methylene carbon (C2) attached to a COOH group. Signals recorded at 76.420ppm, 76.693ppm, 77.011ppm, and 77.330ppm, is the deteriorated chloroform carbon (solvent use). Signal recorded at 130ppm is the CH2=CH2 carbon (C9 and C10). Signal recorded at 178ppm is the COOH carbon (C1) [37,38].
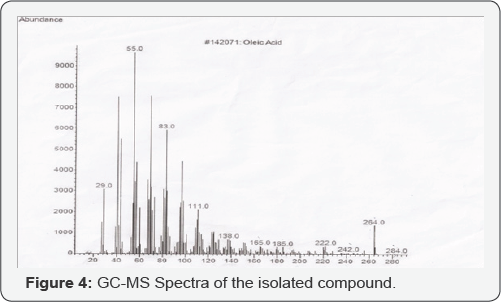
Gas Chromatography/Mass Spectroscopy (GC/MS) Of F5: (Figure 4) The GC/MS of the compound F5 gave the molecular weight of the molecule as 282.4614g/mole; the signal at 222 corresponds to the loss of C4H12. , the signal at 165 corresponds to the loss of C4H8. Radical, the signal at 138 corresponds to the loss of C2H4+, the signal at 111 corresponds to the loss of C2H4+, and the signal at 83 corresponds to the loss of C2H4, the signal at 55 corresponds to the loss of C2H4, the signal at 29 corresponds to the loss of C2H2. Taking all the above information into consideration, compound F5 is interpreted as Cis-9- Octadecenoic acid. It has melting point of 16.3°C [39].
The result of the phytochemical screening carried out on LandolphiaOwariensis and Nuclea latifolia revealed that the plants contained Alkaloids, Tanins, Flavonoids, Saponins, and glycosides. The crude extract from the two plants are found to be very active against salmonella typhi, Shigella dysentriae and Escherichia coli. The result also showed that the ethyl acetate extract of LandolphiaOwariensis was the most active extract.
Structural elucidation of 1HNMR and "R, IR and GC/MS showed that the compound was Cis-9-Octadecenoic acid (with a melting point of 16.3°C). The compound was found to be highly active against the diarrhoea causing microbes, suggesting that it may be used as broad spectrum antibiotics in the treatment of diarrhea [40,41].
References
- Rahmatullah M, Dilara F, Ariful HM, Rownak J, Majeedul H, et al. (2009) A Survey Of Medicinal Plants Used By Kavirajes Of Chalna Area, Khulna District, Bangladesh. Afr J Trad 7(2): 91-97.
- Sofowora A, Eyitope O, Adedeji O (2013) The Role and Place of Medicinal Plants in the Strategies for Disease Prevention.Nigeria.Afr J Tradit Complement Altern Med 10(5): 210-229.
- Galadima A, Kabiru A, Garba ZN (2010) Phytochemical, Antimicrobial and Heavy Metals Studies of Landolphiaowariensis Leaves Extract. International Jour Chem 20: 281-286.
- Tannaz B, Poonam D, Brijesh S, Pundarikakshudu T, Arvind N, et al. (2010) Newer insights into the mechanism of action of Psidiumguajavaleaves in infectious diarrhoea. BMC Complement Altern Med 10: (33).
- Trease GE, Evans WC (2002) Pharmacognosy 15th Edn. Saunders Publishers, London, Pp. 42-44, 221-229.
- Nwogu LA, Igwe CU, Emejulu AA (2008) Effects of Landolphiaowariensis leaf extract on the liver function profile and hemoglobin concentration of albino rats. African Journal of Biochemistry Research 2 (12): 240242.
- Garba S, Salihu L (2009) Antimicrobial properties of some Nigerian medicinal plants, LAP Lambert Academic Publishing p. 77.
- Abdullahi AL, Agho MO, Amo SS, Gamaniel KS, Wambebe C, et al. (2001) Anti-diarrhoeal activity of the aqueous extract of Terminaliaavicennoides roots. Phytother Res 15(5): 431-434.
- Abdullahi JO, Amupitanb AO, Oyewaleb JI, Ibrahim K (2007) An ethno- botanical survey of indigenous flora for treating tuberculosis and other respiratory diseases in Niger State, Nigeria. Journal of Phytomedicine and Therapeutics (12): 1-21.
- Allen SJ, Okok B, Martinez E, Gregorio G, Dans LF, et al. (2004) Probiotics for Treating Infectious Diarrhoea. Cochrane Database System Rev (2): CD003048.
- Balick JM, Cox PA (1996) Plants, People and Culture: the Science of Ethno botany, Scientific American Library, USA p. 228.
- Benoit-Vical F, Valentin A, Cournac V (1998) In vitro Antiplasmodial Activity of Stem and Root Extracts of Nauclealatifolia, SM (Rubiaceae). Journal of Ethnopharmacol 61(3): 173-178.
- Chinedu FA, Nnabuife CC, Ezugwu CO, Uchechukwu A, Utoh N (2012) Antimicrobial Properties of the Chloroform Extract of the Stem Bark of NaucleaLatifolia. International Journal of Pharmacy and Pharmaceutical Sciences 4(2): 744-750.
- Deeni YY, Hussain HSN (1991) Screening for antimicrobial activity and for alkaloids of Nauclealatifolia. Journal of Ethnopharmacol 35(1): 9196.
- Dosso K, Guessan BB, Bidie AP, Gnangoran BN, Meite S, et al. (2012) Anti-diarrhoeal Activity of an Ethanol Extract of the Stem Bark of Piliostigmareticulatum(Caesalpiniaceae) in rats. Afr J Tradit Complement Altern Med 9(2): 242-249.
- Egbung GE, Atangwho IJ, Iwara IA, Odey MO, Ebong PE, et al. (2013) Chemical composition of root and stem bark extracts of Nauclealatifolia. Archives of Applied Science Research 5(3): 193-196.
- Gabriel A, Agbor T, Leopold N, Gogang Y (2004) The anti-diarrhoeal Activity of AlchorneacordifoliaLeaf Extract. Phytother Res 18(11): 873876.
- Garba S, Andrew K, Gayus J (2009) Antibacterial activities of total alkaloids extracted from some medicinal plants. Top-class Journal of Herbal Medicine 2(9).
- Gill L S (1992) Ethno-medical use of plants in Nigeria. Uniben Press Pp. 145-146.
- Gu errant RL, Van-Gilder T, Steiner TS, Theilman MN, Slutsker L, et al. (2001) Practice Guidelines for the Management of Infectious Diarrhoea. ClinInfec Dis 32(3): 331-351.
- Harborne JB, Turner BL (1984) Chemosystematics. London, Academic press. International Journal of Pharmacy and Pharmaceutical Sciences 4: 744-750.
- Haruna B, Adebola O (2011) Preliminary Phytochemical Screening and Antimicrobial Evaluation of three Medicinal Plants used in Nigeria. Afr J Tradit Complement Altern Med 8(4): 387-390.
- Hill AF (1989) Economic Botany: A Text Book of Useful Plants and Plant Products, second edition, McGraw Hill Book Company, Inc, USA P. 560.
- Institute for International Cooperative in Animal Biology 2005. Iowa State University, College of veterinarian Medicine (2005).
- Irvine FR (1961) The woody plants of Ghana. Oxford University press. UK, pp. 26-28.
- Jason BH, Regina CL, Firdausi Q, Edward TR, Stephen BC (2012) Lancet 379: 2466-2476.
- Khalilur, R Soumitra, B Fokhrul Md, Rafikul I, Mohammed AS, et al. (2013) Department of Pharmacy, International Islamic University Chittagong, Bangladesh Department of Pharmacy, University of Rajshahi, Bangldesh Reviewed by Dr. M. AshikMosaddik. Asian Pac J Trop Biomed. 3(8): 639-643.
- Khatun A, Rahman M, Jahan S (2014) Preliminary Phytochemical and Pharmacological Screening of Murrayaexotica Linn. Leaves extract. Oriental Pharmacy and Experimental Medicine 14(3): 223-229.
- Nayeem AA, Khatun A, Rahman MS, Rahman M (2011) Evaluation of Phytochemical and pharmacological properties of Mikaniacordata (Asteraceace) leaves. Journal of Pharmacognosy and phototherapy 3(8): 118-123.
- waogu LA, Igwe KO (2010) Chemical profile of Landolphiaowariensisseed from Ikeduru, Imo State, Nigeria. Int. J Biol Chem Sci 4(4): 1334-1339.
- Ogueke CC, Chikwendu CI, Iwouno JO, Ogbulie JN (2011) Effect of Crude Ethanol Extract of NaucleaLatifoliaon Some Clinical Isolates of Food Importance and Its Toxicological Potentials 3(1): 44-52.
- Olaleye SB, Owoyele VB, Odukanmi AO (2008) Antiulcer and gastric anti-secretory effects of Landolphiaowariensis extracts in rats. Niger Journal Physiol Sci 23(1-2): 23-26.
- Owoyele BY, Olayele SB, Elegba RA (2002) Anti-Inflammatory and Analgesic Activities of Leaf Extract of Landolphiaowariensis. African Journal of Biomedical Research 4(3): 131-133.
- Sarin RV, Bafna PA (2012) Herbal Anti-diarrhoeal: A Review by Rayat Institute of Pharmacy, Rayat and Bahra Campus, Railmajra, Dist Nawanshahar,India. International Journal of Research in Pharmaceutical and Biomedical Sciences 3(2).
- Sarin RV, Bafna PA (2010) Herbal Anti-diarrhoeals: A Review by Rayat Institute of Pharmacy, Rayat and Bahra Campus, India.
- Senthil RD, Rajkumar M, Srinivasan R, Kumarappan C, Meka VS, et al. (2014) Investigation on Antidiarrhoeal Activity of AristolochiaIndicaLinn. Root Extracts in Mice. Afr J Tradit Complement Altern Med. 11(2): 292-294.
- Suleiman MM, Dzenda T, Sani CA (2008) Anti-diarrhoeal activities of the methanol stem-bark extract of AnnonasenegalensisPers. (Annonaceae) Ethnopharmacol 116(1): 125-130.
- Thapar N, Sanderson IR (2004) Diarrhoea in children: an interface between developing and developed countries 363(9409): 641-653.
- Tona L, Kambu K, Ngimbi N (2000) Anti-amoebic and Spasmolytic Activities of Extracts from some Anti-diarrhoeal TraditionalPreparations used in Kinshasa, CongoPhytomedicine 7(1): 31-38.
- (2010) World Health Organization (WHO) (2010) Dr. Claudia Stein Medical Officer (Epidemiologist) Food Safety, Zoonoses and Food borne Diseases.






























