Design, Synthesis of Novel Thieno [2,3-d] derivatives and their Anti-Microbial studies
Virupakshi Prabhakar*1, Kondra Sudhakar Babu2, LK Ravindranath2 and VM Dayalan3
1Faculty of Chemistry, Rgukt-Iiit Ongole, India
2Department of Chemistry, Sri Krishnadevaraya University, India
3Faculty of Engineering Chemistry, Jawaharlal Nehru Technological University-Anantapuramu (JNTU-A), India
Submission: September 10, 2017; Published: September 18, 2017
*Corresponding author: Virupakshi Prabhakar, Faculty of Chemistry, RGUKT-IIIT Ongole, AP, India, Email: viruchem765@gmail.com
How to cite this article: Virupakshi P, Kondra S B, L Ravindranath, V Dayalan .Design, Synthesis of Novel Thieno [2,3-d] derivatives and their Anti- Microbial studies . Organic & Medicinal Chem IJ. 2017; 3(4): 555618. DOI: 10.19080/OMCIJ.2017.03.555618
Abstract
A new series of 4-Substituted/Heterocyclic -N-(4-(4-(trifluoromethyl/Nitro)phenoxy)thieno[2,3-d]pyrimidin-2-yl)benzamide (8 a-j) derivatives were synthesized by a five-step procedure that afforded advantages of mild reaction conditions, simple protocol and good yields. Several Thieno [2,3-d] Pyrimidines have been prepared from methyl 2-aminothiophene-3-carboxylate(1).The structures of the final compounds were confirmed by IR, NMR, EI-MS. The final compounds were screened for their anti-bacterial activity against Staphylococcus aureus (S. aureus) and Bacillus subtilis (B. subtilis) from Gram positive group of bacteria. Pseudomonas aeruginosa (P. aeruginosa) and Escherichia coli (E. coli) from Gram negative group of bacteria. Anti-fungal activity against Aspergillus Niger (A. Niger) and Candida albicans (C. albicans). Anti-bacterial and anti-fungal activities were Evaluated and compared with the standard drugs Such as Amoxicillin & Flucanazole. From anti-bacterial and antifungal activity screening results, it has been observed that compounds 8j, 8i, 8h and 8g possess good activity.
Keywords: Thieno [2, 3-d] Pyrimidine, Acid-amine coupling reaction, 2, 4-di chloro Thieno [2, 3-d] Pyrimidine, Anti-microbial activity
Introduction
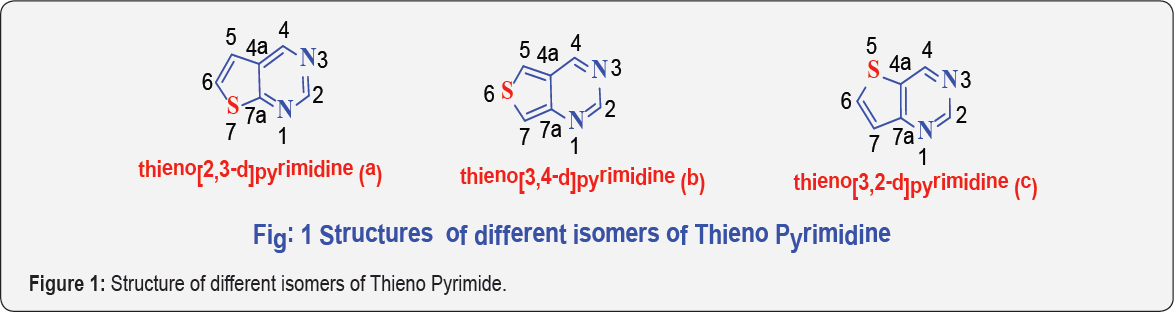
Thieno Pyrimidine is a bi cyclic heterocyclic compound consists of a five membered thiophene ring is fused to a six membered hetero cyclic ring with two nitrogen atoms. The fusion may occur in three different orientations that results in three important types of thienopyrimidines namely;
a) Thieno[2,3-d]Pyrimidine
b) Thieno[3,2-d]Pyrimidine and
c) Thieno[3,4-d]Pyrimidine
Most of the isomeric Thieno Pyrimidine occurs as colored amorphous form, some exists as crystalline form. Synthetic approaches for the construction of a number of Thieno Pyrimidines are well established. There exists three possible types of fusion of thiophene to Pyrimidine ring results in corresponding isomeric Thieno pyrimidines namely; (Figure 1) Thieno[2,3-d] pyrimidines (a), Thieno[3,4-d]pyrimidines (b) and Thieno [3,2-d] pyrimidines (c).
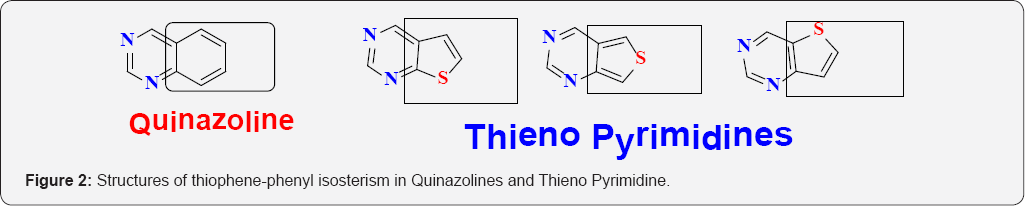
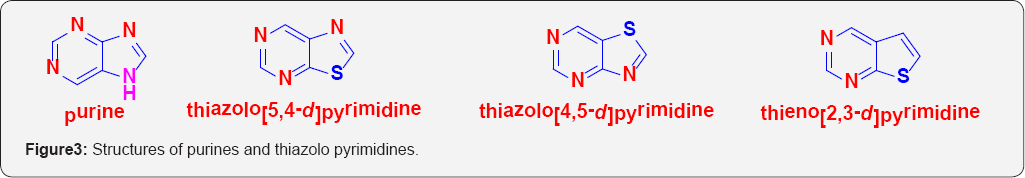
As a logical consequence of thiophene-phenyl isosterism, similarly Thieno pyrimidines can be considered as bio isosteres of quinazolines, which are extensively described in scientific and patent literature as displaying a plethora of biological activities. The synthesis of Thieno pyrimidine derivatives as potential surrogates for the quinazoline core structure has therefore, become a routine strategy in modern drug design and development. Thieno pyrimidines as isosteres of quinazolines are shown here (Figure 2). Thienopyrimidines can also be considered as structural analogues of five-membered heterocycles such as purines and thiazolo-pyrimidines. As interesting anti-HIV activity was discovered within the thiazolo [5,4-d]pyrimidine series, whereas the thiazolo[4,5-d] pyrimidines lack antiretroviral activity. The structures of purines and thiazolo pyrimidines are shown in the following (Figure 3).
Thiophene containing compounds are well known to exhibit various biological effects. Heterocycles containing the Thieno pyrimidine moiety are of interest because of their interesting pharmacological and biological activities [1-3]. They bear structural analogy and iso electronic relation to purine and several substituted Thieno [2,3-d] Pyrimidine derivatives shown to exhibit prominent and versatile biological activities [4,5]. Over the last two decades, many Thieno-pyrimidines have been found to exhibit a variety of pronounced activities. Many of their derivatives have been synthesized as potential anticancer [6], analgesic [7], antimicrobial [8,9] and antiviral agents [10].
Some reviews on Pyrimidine thiones [11] and condensed pyrimidines, namely pyrazolo-pyrimidines [12] and furo- pyrimidines [13]. Thieno-pyrimidines are interesting heterocyclic compounds and a number of derivatives of these compounds display therapeutic activity as antimicrobial [14-17], anti-viral [18-19], anti-inflammatory [20-21], anti-diabetic [22], anti-oxidant [23], anti-tumour [24-28] and anti-cancer agents [29-30], anti-depressant [31], anti-platelet [32], anti-hypertensive [33], herbicidal [34] and plant growth regulatory properties [35].
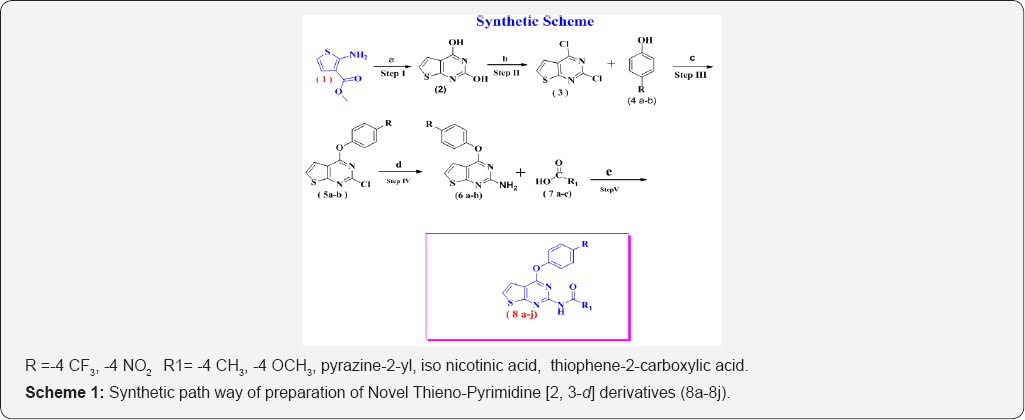
Literature survey revealed that incorporation of different groups in Thieno [2,3-d]pyrimidine Heterocyclic ring enhanced antibacterial and antifungal activity. In the present communication 2,4- di chloro Thieno[2,3-d] Pyrimidine (3) was reacted with various Phenols 4(a-b) in Acetone to form 2-chloro- 4-(4-(trifluoro methyl)phenoxy)Thieno[2,3-d]Pyrimidine (5a-b) , which was further converted in to amine by using aqueous Ammonia to form compounds (6a-b), which was reacted with different Substituted Carboxylic acids (7 a-e) under Acid-amine Coupling reaction reagent to get target compounds (8a-8j). Encouraged by the diverse biological activities of novel Thieno [2, 3-d] Pyrimidine derivatives, it was decided to prepare a new series of derivatives of Thieno [2, 3-d] Pyrimidine as a core unit. The synthesis of the compounds as per the following (Scheme 1) given below. The synthetic route was depicted in scheme 1. The structures of all synthesized compounds were assigned on the basis of IR, Mass, 1H & 13C NMR spectral data analysis. Further these compounds were subjected for antifungal and antibacterial activity.
Materials and Methods
In this Investigation chemicals were purchased from local dealer with Alfa aesar & Avra labs make was used. Chemicals were 99 % pure; purity has been checked by thin layer chromatography and melting point. Conventional method has been used for synthesis of Thieno [2, 3-d] Pyrimidine derivatives. Stirring and reflux method were used for synthesis of Thieno [2, 3- d] Pyrimidine derivatives 8 (a-j) respectively. The synthetic route was depicted in (Scheme 1). The title compounds 8(a-j) were synthesized in five sequential steps using different reagents and reaction conditions, the 8(a-j) were obtained in moderate yields. The structure were established by spectral (IR, JH-NMR, 13C-NMR and mass) data (Scheme 1).
Reagents and Reaction conditions:
a) 5 eq Urea, 190OC, 3 hrs
b) POCl3, Reflux, 6 hrs
c) Sodium hydroxide in water, acetone (1:1 ratio), 080°C; 24 h
d) Aqueous Ammonia,90OC, 6hrs
e) HATU (1-[Bis(dimethylamino)methylene]-1H-1,2,3- triazolo[4,5-fr]pyridinium 3-oxide hexa fluoro phosphate), Hunig,s base (N,N-di isopropyl ethylamine) , DMF, RT,16hrs (Figure 4).

Steps:
a. The base deprotonates the carboxylic acid. The resulting carboxylate anion attacks the electron deficient carbon atom of HATU.
b. The resulting HOBt anion reacts with the newly formed activated carboxylic acid derived intermediate to form an OBt activated ester.
c. The amine reacts with the OBt activated ester to form the amide bond.
Experimental Section
All reactions were carried out under argon in oven-dried glassware with magnetic stirring. Unless otherwise noted, allmaterials were obtained from commercial suppliers and were used without further purification. All solvents were reagent grade. THF was distilled from sodium benzo phenone ketyl and degassed thoroughly with dry argon directly before use. Unless otherwise noted, organic extracts were dried with anhydrous Na2SO4, filtered through a fitted glass funnel, and concentrated with a rotary evaporator (20-30 Torr). Flash chromatography was performed with silica gel (200-300 mesh) by using the mobile phase indicated. The NMR spectra were measured with a 400 MHz Bruker Avance spectrometer at 400.1 and 100.6 MHz, for 1H for 13C, respectively, in CDCl3 solution with tetra methyl silane as internal standard. Chemical shifts are given in ppm (δ) and are referenced to the residual proton resonances of the solvents. Proton and carbon magnetic resonance spectra (1H NMR and 13C NMR) were recorded using tetra methyl silane (TMS) in the solvent of CDCl3-d1 or DMSO-d6 as the internal standard (1H NMR: TMS at 0.00 ppm, CDCl3 at 7.26 ppm ,DMSO at 2.50 ppm; 13C NMR: CDCl3 at 77.16 ppm, DMSO at 40.00 ppm).
Synthesis
General procedure for synthesis of Thieno [2, 3-d] pyrimidine-2, 4-diol [compound (2)]
A mixture of Methyl 2-aminothiophene-3-carboxylate (0.13 mol, 20g) and urea (1 mol, 60g) were mixed with each other, and the mixture was heated for two hours at 200° C. A clear, brown molten mass was formed which solidified upon standing; the solid product was dissolved in warm 1 N sodium hydroxide, and then acidified with 2 N Hydrochloric acid. The crystalline precipitate formed thereby was collected by vacuum filtration and re crystallized from Water, yielding 65% (13.8 gms) of Thieno [2, 3-d] pyrimidine-2, 4-diol.
Yield: 65% (white colour solid);
IR (KBr, cm-1): 3260(N-H Stretching], 3125(Ar C-H], N-O (1140 & 1350), 1680.50 (C=O Stretching).
1H NMR (400 MHz; CDCl3): δH 7.20 (d, 1H, J=7.1HZ), 6.98(1H, d, J=7.1HZ], 7.65(2H,d, J=7.3HZ], 8.15(2H, d, J=7.3HZ], 9.25(1H,bs], 7.91(2H,d, J=7.3 HZ], 8.95 (2H,d, J=7.3 HZ] .
13C NMR (100 MHz; CDCl3): δC 125.5, 128.89, 130.55,133.45,141,149, 155.55, 158.8, 168.34, 172.65.
ESI-MS m/z = 394.465 [M+H] +.
N-(4-(4-nitrophenoxy) Thieno [2,3-d]pyrimidin-2- yl)thiophene-2-carboxamide (8j): (Figure 6.5.10). This compound was obtained as off-yellow solid in 70% yield. m.p. 253-256OC.
IR (KBr, cm-1): 740(-C-Cl), 3110(Ar C-H), 1660 (Ar C=C Stretching).
1H NMR (400 MHz; CDCl3): δH 7.65 (d, 1H, JHH = 6.5 Hz, Ar- H), 7.45 (d, JHH = 6.5 Hz, 1H, Ar-H).
13C NMR (100 MHz; CDCl3): δC 126.92, 123.03, 126.11,153.62, 161.67, 154.75.
GC-MS: RT at 10.968 (100%), m/z = 204(M+H) +, 206(M+2),208(M+4), 9:6:1 it indicates molecule contain two chlorine atoms (Figures 5 & 6).


General procedure for synthesis of 2-chloro-4-(4- (trifluoro methyl)phenoxy)Thieno[2,3-d]Pyrimidine (5a), 2-chloro-4-(4-nitrophenoxy) Thieno [2,3-d] Pyrimidine(5b)
5g (24.5 m.mol) 2, 4-dichloro Thieno [2, 3-d] Pyrimidine (3) dissolved in 50 ml of acetone are slowly added to a solution of 5.32g(130 m.mol) NaOH and 4g(24.5 m.mol) 4-(tri fluoro methyl) phenol(4a)/3.4g 4-nitrophenol (4b) in 100 ml H2O at OOC. After stirring for 24 h at 70OC, the reaction mixture is concentrated under reduced pressure, cooled and the precipitated crude product is filtered off, washed with H2O and dried in vacuum. Purification is performed by flash chromatography (SiO2 Hexane/ EtoAc 2:1).
2-chloro-4-(4-(tri fluoro methyl) phenoxy) Thieno[2,3-d] Pyrimidine (5a):
1H NMR (DMSO-d6) (δ/ppm) δ ppm): 7.25 (d, 1 H, J=7.2HZ), 7.10(1H, d, J=7.2HZ), 7.2 5(1H,d, J=7.1HZ), 7.65(2H, d, J=7.1HZ).
13C NMR (DMSO-d6) (δ/ppm): 123.55, 125.15, 127.65,
128.55, 155.35, 158.55.
IR (KBr, cm-1): Ar stretch C-H (3110), C=N (1646.15), C-F (1345), C-Cl(739),C-O (1362).
ESI-MS m/z 331[M+H]+ .
2- chloro-4-(4-nitrophenoxy) Thieno [2, 3-d] Pyrimidine (5b):
1H NMR (DMSO-d6) (δ/ppm) δ ppm): 7.25 (d, 1 H, J=7.1HZ), 6.95(1H, d, J=7.1HZ), 7.45(2H, d, J=6.7HZ), 8.15(2H, d, J=6.7HZ).
13C NMR (DMSO-d6) (δ/ppm): 123.55, 125.15, 127.65,128.55, 143.55, 155.35, 158.55, 175.55. IR (KBr, cm-1): Ar stretch C-H (3110), N-O (1140 & 1325), C-Cl (730).
ESI-MS m/z 308[M+1].
General procedure for synthesis of 4-(4-(trifluoromethyl)phenoxy)thieno[2,3-d] pyrimidin-2-amine(6a), 4-(4-nitrophenoxy)Thieno[2,3-d]pyrimidin-2-amine (6b)
A solution of 25% aqueous ammonia solution (5 mol) and compounds (5a-5b) (1 mol) was stirred at 90OC for 5h. The precipitate was collected by filtration and washed with water and dried to give compounds (6a-6b).
4-(4-(trifluoromethyl)phenoxy)Thieno[2,3-d ] pyrimidin-2-amine(6a):
1H NMR (DMSO-d6) (δ/ppm) δ ppm): 7.05 (d, 1 H, J=7.1HZ), 7.10(1H, d, J=7.1HZ), 7.45(2H,d, J=7.3HZ), 7.15(2H, d, J=7.3HZ), 6.75(2H,bs).
13C NMR (DMSO-d6) (δ/ppm): 123.55, 125.15, 127.65,128.55, 155.35, 158.55, 163, 173.6.
IR (KBr, cm-1): Ar stretch C-H (3110), C=N (1656.15), C-F (1365), N-H (3339 & 3445),C-O (1352).
ESI-MS m/z 312[M+1].
4-(4-nitrophenoxy)Thieno[2,3-d]pyrimidin-2-amine (6b):
1H NMR (DMSO-d6) (δ/ppm) δ ppm: 7.15 (d, 1 H, J=7.2HZ), 6.90(1H, d, J=7.2HZ), 7.45(2H,d, J=7.3HZ), 8.15(2H, d, J=7.3HZ), 6.95(2H,bs).
13C NMR (DMSO-d6) (δ/ppm): 123.55, 125.15, 127.65,128.55, 145.35, 158.55, 163, 173.6.
IR (KBr, cm-1): Ar stretch C-H (3110), N-O (1140 & 1325), N-H(3339 & 3445).
ESI-MS m/z 289[M+1].
General procedure for synthesis of 4-methyl-N- (4-(4-(trifluoromethyl)phenoxy)thieno[2,3-d] pyrimidin-2-yl)benzamide (8a),4-methoxy-N-(4-(4- (trifluoromethyl)phenoxy)thieno[2,3-d]pyrimidin-2- yl)benzamide(8b),N-(4-(4-(trifluoromethyl)phenoxy) thieno[2,3-d]pyrimidin-2-yl)pyrazine-2-carboxamide (8c), N-(4-(4-(trifluoromethyl)phenoxy)thieno[2,3-d] pyrimidin-2-yl)isonicotinamide(8d), N-(4-(4-(trifluoromethyl)phenoxy)thieno[2,3-d]pyrimidin- 2-yl)thiophene-2-carboxamide (8e), 4-methyl-N- (4-(4-nitrophenoxy)thieno[2,3-d]pyrimidin-2-yl) benzamide(8f),4-methoxy-N-(4-(4- nitrophenoxy) Thieno[2,3-d]pyrimidin-2-yl)benzamide (8g), N-(4- (4-nitrophenoxy)thieno[2,3-d]pyrimidin-2-yl) pyrazine-2-carboxamide (8h), N-(4-(4-nitrophenoxy) Thieno[2,3-d]pyrimidin-2-yl)iso nicotinamide (8i), N-(4-(4-nitrophenoxy)thieno[2,3-d]pyrimidin-2-yl) thiophene-2-carboxamide (8j):
To a solution ofVarious Substituted Acids (7a-7e) (10.2 m.mol) in DMF (5v), HATU (10 m.mol), Hunig,s base (N,N-di isopropyl ethylamine, DIPEA) (20 m.mol), Stir at RT for 10 min under Nitrogen atmosphere, Then add 4-(4-(trifluoromethyl)phenoxy) thieno[2,3-d]pyrimidin-2-amine(6a), 4-(4-nitrophenoxy)Thieno[2,3-d]pyrimidin-2-amine (6b) (10.00 m.mol) at RT for 16 hrs, Then Reaction mixture was diluted with Ice Cold Water, Filtered the obtained Solid and Dried, Finally Purified by Flash Column Chromatography.
4-methyl-N-(4-(4-(trifluoromethyl)phenoxy) thieno[2,3-d]pyrimidin-2-yl)benzamide (8a): (Figure 6.5.1). This compound was obtained as off-white solid in 75% yield. M.p. 236-238OC.

IR (KBr, cm-1): 3243(N-H Stretching), 3110(Ar C-H), C-F (1365), 1695.20 (C=O Stretching).
1H NMR (DMSO-d6) (δ/ppm) δ ppm 2.35 (3H,S),7.15 (d, 1 H, J=7.1HZ), 7.10(1H, d, J=7.1HZ), 7.45(2H,d, J=7.3HZ), 7.15(2H, d, J=7.3HZ), 9.15(1H,bs), 7.91(2H,d, J=7.3 HZ), 7.35 (2H,d, J=7.3 HZ).
13C NMR (DMSO-d6) (δ/ppm): 23, 123.55, 125.15, 127.65,128.55, 145.35, 158.55, 163, 173.6.
13C NMR (100 MHz; CDCl3): δC 28, 105.73, 125.5, 128.89,130.55, 133.45,149, 158.8, 165.34.
ESI-MS m/z 430[M+1].
4-methoxy-N-(4-(4-(trifluoromethyl)phenoxy) thieno[2,3-d]pyrimidin-2- yl)benzamide(8b): (Figure 6.5.2). This compound was obtained as off-white solid in 80% yield. m.p. 247-249OC.

IR (KBr, cm-1): 2920(SP3C-H), 3243(N-H Stretching), 3110(Ar C-H), 1687.20 (C=O Stretching).
1H NMR (400 MHz; CDCl3): δH 7.15 (d, 1H, J=7.1HZ), 7.10(1H, d, J=7.1HZ), 7.45(2H,d, J=7.3HZ), 7.15(2H, d, J=7.3HZ), 9.15(1H,bs), 7.91(2H,d, J=7.3 HZ), 7.35 (2H,d, J=7.3 HZ).
13C NMR (100 MHz; CDCl3): δC 58.88, 105.73, 125.5, 128.89, 130.55, 133.45,141,149, 158.8, 168.34.
ESI-MS m/z = 446.465 [M+H] +.
N-(4-(4-(trifluoromethyl)phenoxy)thieno[2,3-d] pyrimidin-2-yl)pyrazine-2- carboxamide (8c): (Figure 6.5.3). This compound was obtained as off-yellow solid in 80% yield. m.p. 147-149OC.
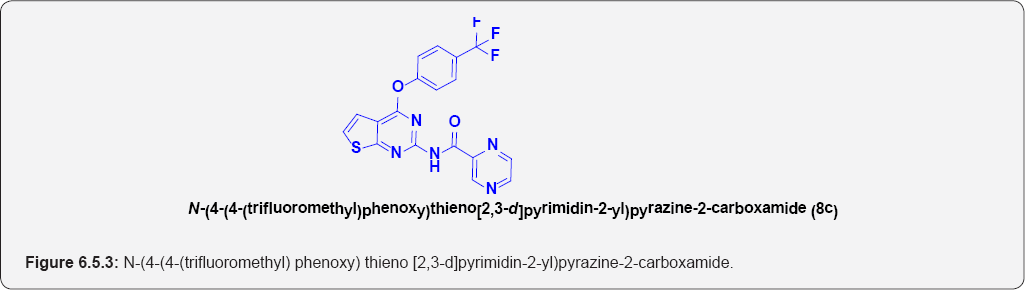
IR (KBr, cm-1): 3234(N-H Stretching), 3105(Ar C-H), 1690.20 (C=O Stretching).
1H NMR (400 MHz; CDCl3): δH 7.25 (d, 1H, J=7.1HZ), 6.95(1H, d, J=7.1HZ), 7.65(2H,d, J=7.3HZ), 7.15(2H, d, J=7.3HZ), 9.05(1H,bs), 9.91(1H,d, J=2.3 HZ), 9.15 (1H,d, J=7.3 HZ), 8.95(1H, d, J=7.3 HZ).
13C NMR (100 MHz; CDCl3): δC 125.5, 128.89, 130.55,133.45,141,149, 155.55,158.8, 168.34, 172.65.
ESI-MS m/z = 418.465 [M+H] +.
N-(4-(4-(trifluoromethyl)phenoxy)thieno[2,3-d] pyrimidin-2-yl)isonicotinamide(8d): (Figure 6.5.4). This compound was obtained as off-yellow solid in 75% yield. m.p. 182-183OC.

IR (KBr, cm-1): 3264(N-H Stretching), 3115(Ar C-H), 1685.50 (C=O Stretching).
1H NMR (400 MHz; CDCl3): δH 7.20 (d, 1H, J=7.1HZ), 6.98(1H, d, J=7.1HZ), 7.65(2H,d, J=7.3HZ), 7.15(2H, d, J=7.3HZ), 9.45(1H,bs), 7.91(2H,d, J=2.3 HZ), 8.95 (1H,d, J=7.3 HZ) .
13C NMR (100 MHz; CDCl3): δC 125.5, 128.89, 130.55,133.45,141,149, 155.55, 158.8, 168.34, 172.65.
ESI-MS m/z = 417.465 [M+H] +.
N-(4-(4-(trifluoro methyl) phenoxy)thieno[2,3-d] pyrimidin-2-yl)thiophene-2-carboxamide (8e): (Figure 6.5.5). This compound was obtained as off-white solid in 70% yield. m.p. 153-156OC.
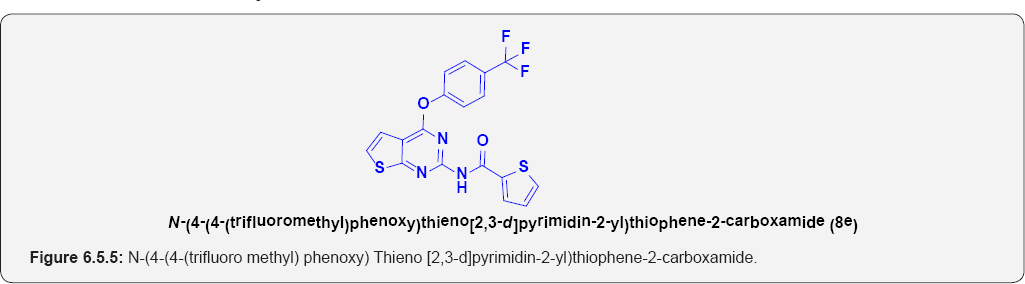
IR (KBr, cm-1): 3264(N-H Stretching), 3105(Ar C-H), 1690.50 (C=O Stretching).
1H NMR (400 MHz; CDCl3): δH 7.20 (d, 1H, J=7.1HZ), 6.98(1H, d, J=7.1HZ), 7.45(2H,d, J=7.3HZ), 7.15(2H, d, J=7.3HZ), 9.35(1H,bs), 8.30(1H,d, J=6.8HZ), 8.15 (1H,d, J=6.8 HZ), 7.35(1H,t, J=6.8 HZ) .
13C NMR (100 MHz; CDCl3): δC 125.5, 128.89, 130.55,133.45,141,149, 155.55, 158.8, 168.34, 172.65.
ESI-MS m/z = 422.465 [M+H] +.
4- methyl-N-(4-(4-nitrophenoxy) Thieno [2,3-d] pyrimidin-2-yl) benzamide (8f): (Figure 6.5.6). This compound was obtained as Pale-yellow solid in 75% yield. m.p. 223-225OC.
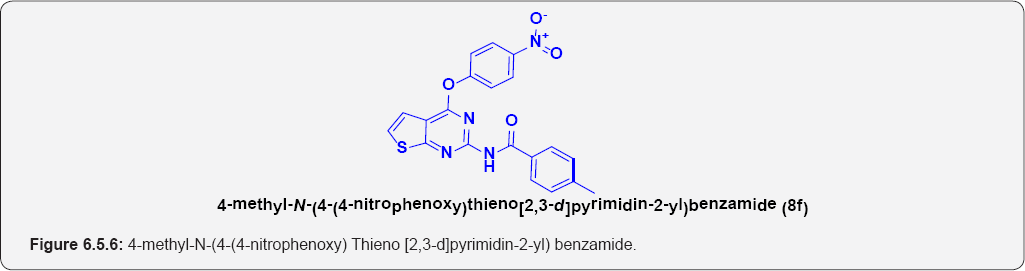
IR (KBr, cm-1): 3264(N-H Stretching), 3105(Ar C-H), N-O (1140 & 1325), 1690.50 (C=O Stretching).
1H NMR (DMSO-d6) (δ/ppm) δ ppm 2.35 (3H,S),7.15 (d, 1 H, J=7.1HZ), 7.10(1H, d, J=7.1HZ), 7.45(2H,d, J=7.3HZ), 8.15(2H, d,J=7.3HZ), 9.15(1H,bs), 7.91(2H,d, J=7.3 HZ), 7.35 (2H,d, J=7.3 HZ).
13C NMR (DMSO-d6) (δ/ppm): 23, 123.55, 125.15, 127.65,128.55, 145.35, 158.55, 163, 173.6.
ESI-MS m/z = 407.26 [M+H] +.
4-methoxy-N-(4-(4-nitrophenoxy)thieno[2,3-d] pyrimidin-2-yl)benzamide (8g): (Figure 6.5.7). This compound was obtained as yellow solid in 73% yield. m.p. 252-253OC.
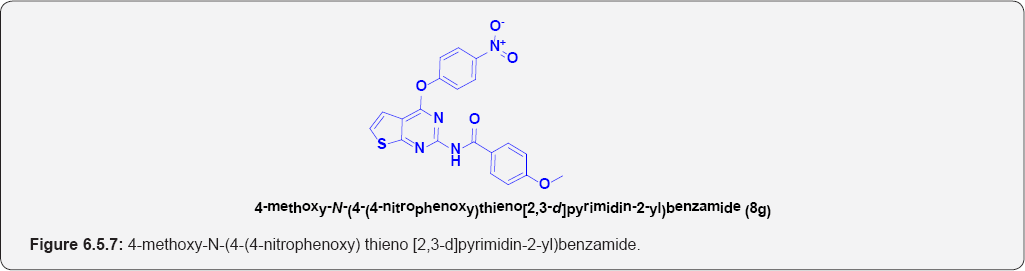
IR (KBr, cm-1): 3260(N-H Stretching), 3105(Ar C-H), N-O [1148 & 1320], 1695 (C=O Stretching].
1H NMR (DMSO-d6] [δ/ppm] δ ppm 3.85 [3H,S],7.15 [d, 1 H, J=7.1HZ), 7.10(1H, d, J=7.1HZ), 7.4S(2H,d, J=7.3HZ), 8.1S(2H, d, J=7.3HZ), 9.1S(1H,bs), 7.90(2H,d, J=7.3 HZ), 7.2S (2H,d, J=7.3 HZ).
13C NMR (DMSO-d6) (δ/ppm): 58, 123.55, 125.15, 127.65,128.55, 145.35, 158.55, 163, 173.6.
ESI-MS m/z = 421.26 [M-H] +.
N-(4-(4-nitrophenoxy)thieno[2,3-d]pyrimidin-2-yl) pyrazine-2-carboxamide (8h): (Figure 6.5.8). This compound was obtained as pale-yellow solid in 80% yield. m.p. 245-247OC.
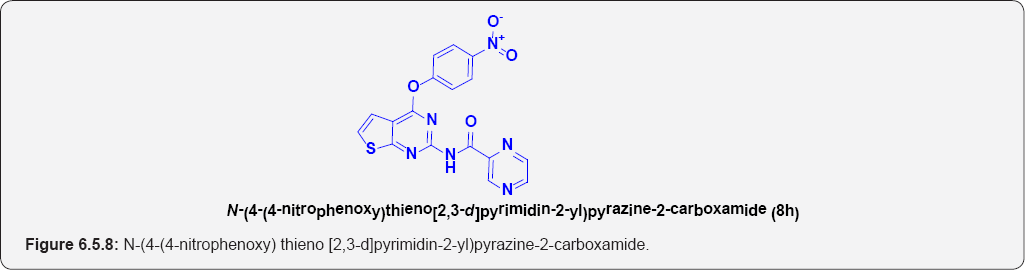
IR (KBr, cm-1): 3240(N-H Stretching), 3110(Ar C-H), N-O [1158 & 1340], 1685.20 [C=O Stretching].
1H NMR (400 MHz; CDCl3): δH 7.25 [d, 1H, J=7.1HZ], 6.9S(1H, d, J=7.1HZ), 7.55(2H,d, J=7.3HZ), 8.1S(2H, d, J=7.3HZ), 9.15(1H,bs), 9.91(1H,d, J=2.3 HZ), 9.1S (1H,d, J=7.3 HZ), 8.9S(1H,d, J=7.3 HZ).
13C NMR (100 MHz; CDCl3): δC 125.5, 128.89, 130.55,133.45,141,149, 155.55,158.8, 168.34, 172.6S.
ESI-MS m/z = 395.465 [M+H] +.
N-(4-(4-nitrophenoxy) Thieno [2,3-d]pyrimidin-2-yl)iso nicotinamide (8i): (Figure 6.5.9). This compound was obtained as off-yellow solid in 78% yield. m.p. 232-233OC.
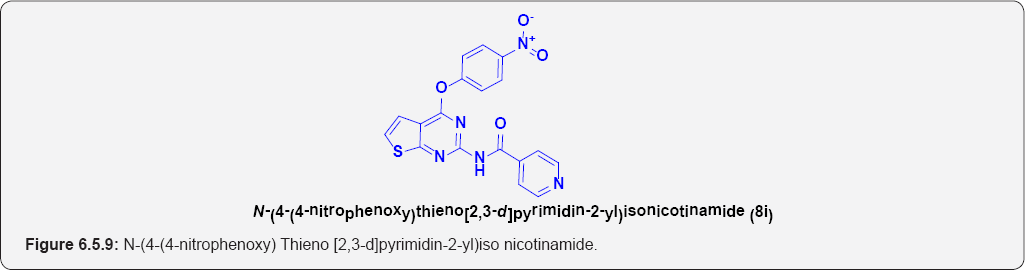
IR (KBr, cm-1): 3260(N-H Stretching], 3125(Ar C-H], N-O (1140 & 1350), 1680.50 (C=O Stretching).
1H NMR (400 MHz; CDCl3): δH 7.20 (d, 1H, J=7.1HZ), 6.98(1H, d, J=7.1HZ], 7.65(2H,d, J=7.3HZ], 8.15(2H, d, J=7.3HZ], 9.25(1H,bs], 7.91(2H,d, J=7.3 HZ], 8.95 (2H,d, J=7.3 HZ] .
13C NMR (100 MHz; CDCl3): δC 125.5, 128.89, 130.55,133.45,141,149, 155.55, 158.8, 168.34, 172.65.
ESI-MS m/z = 394.465 [M+H] +.
N-(4-(4-nitrophenoxy) Thieno [2,3-d]pyrimidin-2- yl)thiophene-2-carboxamide (8j): (Figure 6.5.10). This compound was obtained as off-yellow solid in 70% yield. m.p. 253-256OC.
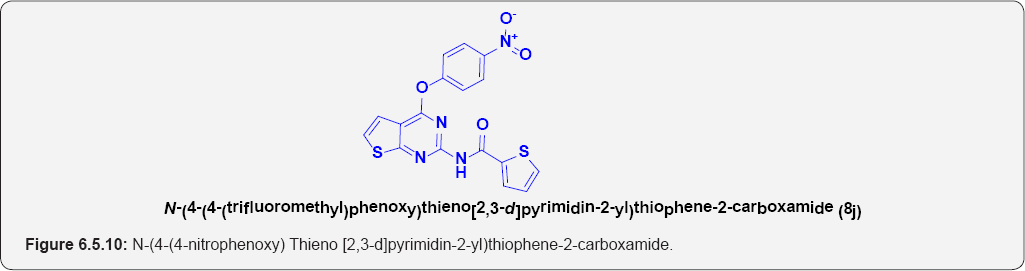
IR (KBr, cm-1): 3264(N-H Stretching], 3105(Ar C-H], N-O (1150 & 1360), 1690.50 (C=O Stretching).
1H NMR (400 MHz; CDCl3): δH 7.20 (d, 1H, J=7.1HZ),6. 95(1H, d, J=7.1HZ], 7.45(2H,d, J=7.1HZ], 8.15(2H, d, J=7.1HZ], 9.38(1H,bs], 8.30(1H,d, J=6.8HZ], 8.15 (1H,d, J=6.8 HZ], 7.35(1H,t, J=6.8 HZ] .
13C NMR (100 MHz; CDCl3): δC 125.5, 128.89, 130.55,133.45,141,149, 155.55, 158.8, 168.34, 172.65.
ESI-MS m/z = 399.465 [M+H] +.
Biological Activity
Antibacterial studies
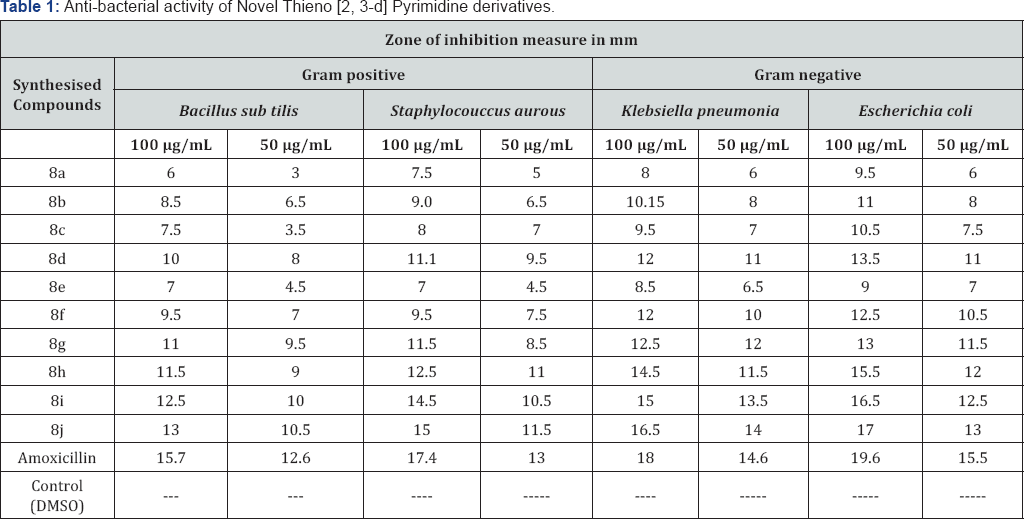
The newly prepared compounds were screened for their antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumonia and Escherichia coli (clinical isolate] bacterial strains by disc diffusion method [36,37]. Standard inoculums (1-2x107 c.f.u. /ml 0.5 McFarland standards) were introduced on to the surface of sterile agar plates, and a sterile glass spreader was used for even distribution of the inoculums. The disks measuring 6 mm in diameters were prepared from what man no. 1 filter paper and sterilized by dry heat at 140°C for 1 h. The sterile disks previously soaked in a known concentration of the test compounds were placed in nutrient agar medium. Solvent and growth controls were kept. Amoxicillin (30 μg) was used as positive control and the disk poured in DMSO was used as negative control and the test compounds were dissolved in DMSO at concentration of 100 and 50 μg/ml. The plates were inverted and incubated for 24 h at 37°C. The susceptibility was assessed on the basis of diameter of zone of inhibition against Gram-positive and Gram-negative strains of bacteria. Inhibition of zone of measured and compared with controls. The bacterial zone of inhibition values are given in (Table 1). The order of activity was 8j>8i>8h>8g>8d>8f>8b>8c>8e>8a (Table 1).
Antifungal Studies
The newly prepared compounds were screened for their antifungal activity against Candida albicans and Aspergillus flavus in DMSO by agar diffusion method [38]. Sabourauds agar media was prepared by dissolving peptone (1 g), D-glucose (4 g) and agar (2 g) in distilled water (100 ml) and adjusting pH 5.7. Normal saline was used to make suspension of corresponding species. Twenty milliliters of agar media was poured into each Petri dish. Excess of suspension was decanted and the plates were dried by placing in an incubator at 37°C for 1 h using an agar punch, wells were made and each well was labeled. A control was also prepared in triplicate and maintained at 37°C for 3-4 days. The fungal activity of each compound was compared with Flucanazole as a standard drug. Inhibition zone were measured and compared with the controls. The fungal zone of inhibition values are given in (Table 2).
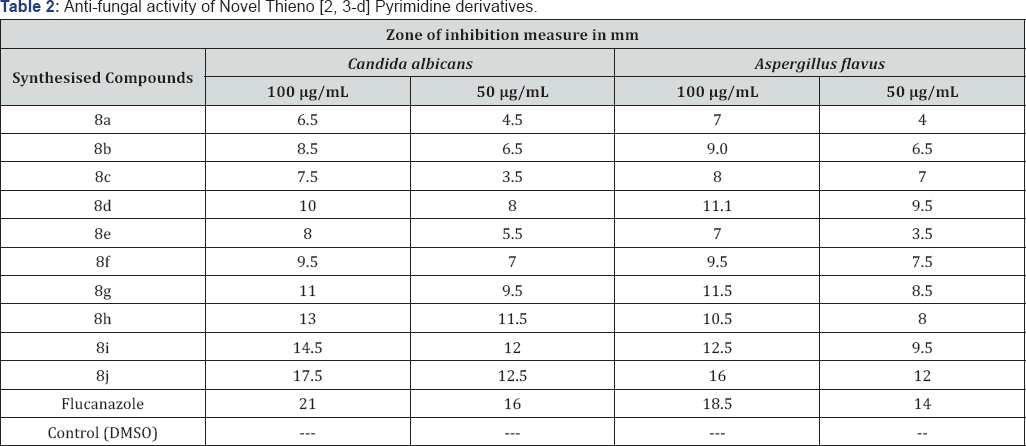
Result and Discussions
Chemistry
The reaction sequences Employed for synthesis of title compounds are shown in (Scheme 1). In the present work, the starting Thieno [2,3-d]pyrimidine-2,4-diol(2) was prepared from methyl 2-aminothiophene-3-carboxylate (1) and Urea According to the reported procedure [39]. Next Step 2 is 2,4-dichloro Thieno[2,3-d]pyrimidine (3) was prepared by using POCl3 at reflux for 6 hrs According to the reported procedure [40]. The 2,4-dichlorothieno[2,3-d]pyrimidine (3) was Coupling with different Phenols (4 a-b) in Acetone at 70oC to get compounds 5(a-b) According to the reported procedure [41]. Which is further treatment with Aqueous Ammonia at 90OCAccording to the reported procedure [42]? which on further treatment with different Carboxylic acids (7a-e) to get target novel Thieno [2, 3- d] pyrimidine derivatives (8a-j) According to the reported procedure [42]. All compounds displayed IR, 1H and 13C NMR and mass spectra consistent with the assigned structures.1H NMR and IR spectrum of compounds (8 a-j) showed singlet at 2.3 ppm, 3.8 ppm are due to the aromatic methyl group protons and Aromatic methoxy group protons. The most characteristic IR absorption bands are at 3340 cm-1 (-NH), 760 cm-1 (C-Cl), 1150 & 1350 (N-O) cm-1 and 3320 &3250cm-1 (N-H Stretching in Amine group). The mass spectra of all the final derivatives showed comparable molecular ion peak with respect to molecular formula.
Anti-microbial studies
The newly synthesized compounds (8a-j) were screened for their in-vitro anti-bacterial activity against Bacillus subtilis, Staphylococcus aurous, Klebsiella pneumonia and Escherichia coli using Amoxicillin as standard by disc diffusion method (zone of inhibition. The test compounds were dissolved in dimethylsulfoxide (DMSO) at concentrations of 50 and 100 μg/ml. The antibacterial screening revealed that all the tested compounds showed good inhibition against various tested microbial strains compared to the standard drug. Along with he synthesized compounds 8j, 8i, 8h, 8g were found to be more active against tested bacterial strains as compared to the standard.
Conclusion
The research study reports the successful synthesis and anti-microbial activity of novel Thieno [2,3-d] Pyrimidine as a core unit . The anti-microbial activity study revealed that all the tested compounds showed good antibacterial and antifungal activities against pathogenic strains. Compounds 8j, 8i, 8h and 8g exhibited more potent anti-microbial activity of all tested pathogenic strains. Few of synthesized compounds might be useful as antimicrobial agents in future. These novel Thieno [2,3-d] Pyrimidine derivatives have proved to be promising candidates for further efficacy evaluation. On the basis of their activity, these derivatives were identified as viable leads for further studies.
Acknowledgment
Authors are thankful to Prof. K. Sudhakar Babu, Registrar, Sri Krishnadevaraya University for Encouraging & facilities of IR Spectra, 1H NMR & 13C NMR for characterization of Novel Synthesized compounds.
References
- Ibrahim YA, Elwahy AHM (1996) Thieno pyrimidines: Synthesis, reactions, and biological activity. Adv Heterocycl Chem 65: 235.
- Ismail KA, Aboulwafa OM, Koreish E (1995) Synthesis and antimicrobial activity of some tetramethylenethieno [2,3-d]pyrimidine derivatives. Farmaco 50(9): 611-616.
- Hammam AG, Sharaf M, Abdelhafez NA (2001) Synthesis and anticancer activity of pyridine and thiazolopyrimidine derivatives using 1-ethylpiperidone as a syntho. Indian J Chem 40B: 213-221.
- Aymn E, Rashad Ahmed H, Shamroukh Randa E, AbdelMegeid, Wael A, et al. (2010) synthesis, reactions and antimicrobial evaluation of some polycondensed-thieno-pyrimidines derivatives. Synth Commun 1(40): 1149-1160.
- Rashad AE, Shamroukha AH, Sayed HH, Awad SM, Abdelwahed AM (2011) Some novel thienopyrimidine nucleoside analogs: Synthesis and in vitro anti-microbial evaluation. Synth Commun 41: 652.
- Amr AE, Mohamed AM, Mohamed SF, AbdelHafez NA, Hammam AG(2006) Synthesis and anticancer activities of new pyridine, pyran, and pyrimidine derivatives fused with nitrobenzosubetrone moiety. Bioorg Med Chem 14: 5481.
- Amr AE, Hegab MI, Ibrahim AA, Abdalah MM (2003) Synthesis and reactions of some fused oxazinone, pyrimidinone, thiopyrimidnone, and triazinone derivatives with thiophene ring as analgesic, anticonvulsant, and anti Parkinsonian agents. Monatsh Chem 134: 1395.
- Hassan NA, Hegab MI, Rashad AE, Fahmy AA, Abdel-Megeid FME (2007) Synthesis and antimicrobial activity of some cyclic and acyclic nucleosides of Thieno[2,3-d]pyrimidines. Nucleosides Nucleotides 26: 379-390.
- Elmahdy KM, Elkazak AM, Abdel Megid M, M Seada, Osama F Mohamed (2013) Synthesis, Characterization and biological evaluation of some new Thieno[2,3-d]pyrimidine derivatives. Advances in Chem 5: 581591.
- Rashad AE, Ali MA (2006) Synthesis and antiviral screening of some Thieno [2,3-d] pyrimidine nucleosides. Nucleotides 25: 17-28.
- Abdel Megid M, Elmahdy KM, Rashad AE (2013) Synthesis and application of Pyrimidine thiones. Global J Science F Res 13: 7.
- Shamroukh AH, Rashad FE AR, Abdel Megid M (2014) The Chemistry of pyrazolo pyrimidines and their applications. Organic Chemistry AI J 10: 224-250.
- Abdel Megid M, Elkazak A M, Seada M, Mohamed OF (2013) Synthesis of furo pyrimidine derivatives. J Advances in Chem 3: 229.
- Kerru N, Settypalli T, Nallapaneni H, Chunduri VR (2014) Novel thienopyrimidines derivatives containing 1,2,4-triazoles and 1,3,4-oxadiazoles as potent antimicrobial activity. Med Chem 4(9): 623-629.
- Mahmoud MR, Abu El-Azm FS, Ali AT, Ali YM (2015) Design, synthesis, and antimicrobial evaluation of novel thienopyrimidines and triazolothienopyrimidines. Synth Commun 45(8): 982-992.
- Khan AY, Kalashetti MB, Belavagi NS, Deshapa-nde N, Khazi IAM (2014) Synthesis, characterization and biological evaluation of novel thienopyrimidines and triazolothienopyrimidine derivatives as antitubercular and antibacterial agents. Am J Pharm Tech Res 4: 283.
- Ramamurthy S, Jayachandran E (2015) Synthesis and characterization of some new 2-methyl-3-Nsubstitutedimino-5,6- tetramethylenethieno[2,3-d] pyrimidin(3H)-4-ones for antibacterial and antifungal screening. Hygeia J D Med 7: 38.
- Kharizomenova IA, Grinev AN, Samsonova NV, Panisheva EK, Kaplina NV, et al. (1981) Functional derivatives of thiophene XX. Synthesis and antiviral activity of 3aminothieno [2,3-d]pyrimidines. Pharm Chem J 15: 645.
- Rashad AE, Shamroukh AH, Abdel-Megeid RE, Mostafa A, El-Shesheny R, et al. (2010) Synthesis and screening of some novel fused thiophene and thienopyrimidine derivatives for anti-avian influenza virus (H5N1) activity. Eur J Med Chem 45(11): 5251-5257.
- Alagarsamy V, Meena S, Ramseshu KV, Solomon VR, Thirumurugan K, et al. (2006) Synthesis, analgesic, anti-inflammatory, ulcerogenic index and antibacterial activities of novel 2-methylthio-3substituted-5,6,7,8- tetrahydrobenzo(b)Thieno [2,3d]pyrimidin-4-(3H)-ones. Eur J Med Chem 41: 1293.
- El-Gazzar ABA, Hussein HAR, Hafez HN (2007) Synthesis and biological evaluation of Thieno[2,3-d] pyrimidine derivatives for antiinflammatory, analgesic and ulcerogenic activity. Acta Pharm 57(4): 395-411.
- Deng JF, Peng L, Zhang GC, Lan XB, Li CF, et al. (2011) The highly potent and selective dipeptidyl peptidase IV inhibitors bearing a thienopyrimidine scaffold effectively treat type 2 diabetes. H Eur J Med Chem 46(1): 71-76.
- Nagaraju K, Harikrishna N, Vasu K, Rao CV (2015) Synthesis and biological activity of novel bis and mono heterocycles of thienopyrimidine derivatives. Indo Am J Pharm Res 5(4): 1604-1612.
- Guo Y, Li J, Ma J, L Yu Z, Wang H, et al. (2015) Synthesis and anti-tumor activity of aaminophosphonate derivatives containing thieno[2,3-d] pyrimidines. Chin Chem Lett 26: 755.
- Kaizhen S, Junjie M, Xiao W, Ping G, Yanfang Z (2014) Synthesis and antitumor activities of novel 4morpholino thieno[2,3-d]pyrimidine derivatives. Chem Res Chin Univ 26(06): 755-758.
- Zhu W, Chen C, Sun C, Xu S, Wu C, et al. (2015) Design, synthesis and docking studies of novel thieno pyrimidine derivatives bearing chromone moiety as mTOR/PI3Ka inhibitors. Eur JMed Chem 93: 6474.
- Becker T, Sellmer A, Eichhorn E, Pongratz H, Schächtele C, et al. (2012) Novel inhibitors of Epidermal growth receptor: (4-(Arylamino)- 7-Hpyrrolo[2,3-d]pyrimidin-6-yl)(1H-indol-2-yl)methanones and (1H-indol-2-yl)(4-(phenylamino)thieno[2,3-d]pyrimdin6-yl) methanones. Bioorg Med Chem Lett 20(1): 125-136.
- Ni Y, Gopalsamy A, Cole D, Hu Y, Denny R, et al. (2011) Identification and SAR of a new series of thieno[3,2-d]pyrimidines as Tpl2 kinase inhibitors. Bioorg Med Chem Lett 21(19): 5952-5956.
- Kandeel MM, Rafaat HM, Kassab AE, Shahin IG, Abdelghany TM (2015) Synthesis, anticancer activity and effects on cell cycle profile and apoptosis of novel thieno[2,3-d]pyrimidine and Thieno[3,2-e] thiazolo[4,3-c] pyrimidine derivatives. Eur J Med Chem 90: 620-632.
- El-Ansary AK, Kamal AM, Al-Ghorafi MA (2014) Synthesis and evaluation of 4-anilinoquinazoline bioisosteres as potential anti-breast cancer agents. Eur J Med Chem 86: 202-210.
- George T, Kaul CL, Grewal RS, Tahilramani R (1971) Antihypertensive and monoamine oxidase inhibitory activity of some derivatives of 3-formyl-4-oxo-4Hpyrido[1,2-a]Pyrimidine. J Med Chem 14(10): 909 913.
- Bruno O, Brullo C, Ranise A, Schenone S, Bondavalli F, et al. (2001) Synthesis and pharmacological evaluation of 2,5-cycloamino-5H[1] benzopyrano[4,3-d]pyrimidines endowed with in vitro anti platelet activity. Bioorg Med Chem Lett 11(11): 1397-1400.
- Kim Y, Kim M, Park M, Tae J, Baek D, et al. (2015) Synthesis of novel dihydropyridothienopyrimidin-4,9-dione derivatives. Molecules 20(3): 5074-5084.
- Liu H, Wang HQ, Liu ZJ (2007) Synthesis and herbicidal activity of novel pyrazolo [3,4-d]pyrimidin-4-one derivatives containing aryloxy phenoxy propionate moieties. Bioorg Med Chem Lett 17(8): 22032209.
- Wang JM, Asami T, Yoshida S, Murofushi N (2001) Synthesis and biological evaluation of 5-substituted pyrimidines as potential plant growth regulators that inhibit brassinosteroids biosynthesis. Biosci Biotechnol Biochem 65: 817.
- Cruickshank R, Duguid JP, Marmion BP, Swain RHA (1975) In Medicinal microbiology. (12) Churchill Livingstone, UK.
- Collins AH (1976) Microbiological methods (2) Butterworth, London, UK.
- Varma RS (1998) Anti-fungal agents: past, present and future prospects, National Academy of Chemistry & Biology, India.
- Jifeng deng, Lipeng, Guicheng zhang (2011) The highly potent and selective di peptidyl Peptidase IV Inhibitors bearing a Thieno[2,3-d] Pyrimidine Scaffold effectively treat type 2 diabets. European Journal of Medicinal Chemistry 46: 71-76.
- Prabhakar V, Sudhakar B, Maddula SR, Parandhama G, Latha J, et al. (2016) Synthesis, Structural Elucidation of Novel Thieno [2,3-d] Pyrimidine Core Unit Containing 1,2,4-Triazoles and Thiophenes as Potent Antimicrobial Activity. Organic Chem Curr Res.
- Miller John F, Andrews C Webster, Brieger Michael, FurfineEric S Hale, Michael R, et al. (2006) Bioorganic and Medicinal Chemistry Letters 16(7): 1788-1794.
- Virupakshi Prabhakar, Kondra Sudhakar Babu, LK Ravindranath, J Latha (2017) Design, Synthesis, Characterization and Biological Activity of Novel Thieno[2,3-d]pyrimidine Derivatives. Indian Journal of Advances in Chemical Science 5(1): 30-42.






























