Novel route for Synthesis of Antihypertensive activity of Tetrazole analogues as a Carbamate and Urea Derivatives.
Ramakrishna Vellalacheruvu*, R. Sai Leela, Dr L. K. Ravindranath, Mastanaih Thummisetty
Department in Chemistry, Sri Krishnadevaraya University, Anantapur, Andhra Pradesh, India GVKBio Science Pvt. Ltd., IDA, Nacharam, Secundrabad, Telangana, India
Submission: August 21, 2017; Published: September 06, 2017
*Corresponding author: Ramakrishna Vellalacheruvu, Department of Chemistry, SK University, Anantapur (A.P), India, Tel: 9493268448; Email: vellala143@gmail.com
How to cite this article: Ramakrishna V, R Sai Leela, LK Ravindranath, Mastanaih T. Novel Route for Synthesis of Antihypertensive Activity of Tetrazole Analogues as a Carbamate and Urea Derivatives. . Organic & Medicinal Chem IJ. 2017; 3(2): 555609. DOI: 10.19080/OMCIJ.2017.03.555609.
Abstract
The Novel route was developed for synthesis of high potential tetrazole carbamate and urea derivatives by using conventional methods. (trifluoromethyl)phenyl)qumohne-5-carboxamide (3) was converted into chloroamidine derivative by using POC13 and DMF (cat), then treated with sodium azide by [3 + 2] cycloaddition to give 8-(benzyloxy)-5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5-yl) quinoline (5). The tetrazolidine compound was debenzylated, then Alkylation with Ethyl Bromo acetate and converted to acid (8) by hydrolysis with LiOH. The acid was converted to acid azide by using DPPA, and then Treated with Alcohols and Amine to give substituted Carbamates and urea derivatives by using Curtius rearrangement.
Keywords: Tetrazolidine, Diphenyl Phosphoryl Azide, [3+2] Cycloaddition Reaction, Curties Re-Arrangement, Combi- Flash Chromatography.
Abbreviations: API: Active Pharmaceutical Drug Intermediates; SSNRI: Selective Serotonin And Nor Epinephrine Reuptake Inhibitors; RAS: Renin Angiotensin System; ABDD: Analogue Based Drug Discovery; RAS: Renin Angiotensin System
Introduction
Tetrazole analogues have a potential pharmacological activity in medicinal chemistry division. Several Active Pharmaceutical Drug Intermediates (API) of tetrazole derivatives played their role in pharmaceutical and agrochemical region. These compounds act as multidimensional biological active drug candidates such as inhibition of Angiotensin (ATI), Angiotensin (AT2) receptor (Hypertension) [1], antifungal & antibacterial [2], corrosion inhibitor [3], anti-inflammatory [4], anticancer [5], [6,7], antioxidant [6], antifungal, analgesic [8], Antiviral [9], protein arginine deiminase inhibitor [10], antimicrobial [11,12], Anti allergic, Dual Selective Serotonin And Nor Epinephrine Reuptake Inhibitors (SSNRls) and HIV inhibitors. When Drug model designing, tetrazole nucleolus consider as a co-sister of carboxylic acid and amide derivatives. The pka value of tetrazole is correlated with amide and acid functional groups. The introduction of tetrazole ring in drug substrate prominently increases their bio-availability and prolonging biological action and also avoids acute toxicity of drug. In Analogue-Based Drug Discovery (ABDD), introduce tetrazole nucleolus as an important descriptor. Several research works is progress based on synthesis of tetrazole amino acid analogues, and nucleotide and nucleoside analogues instead of acid and amide functional group.
Many Tetrazole analogs were available for treatment of hypertension, such as Losartan potassium, Valsartan, Irbesartan, Candesartan and Olmesartan medoxonil. These drugs played a vital role to inhibit Angiotensin converting enzyme. They block ATj & AT2 receptors which is located in kidney, heart, vascular smooth muscle cells, brain, and adrenal glands. The Renin Angiotensin System (RAS) is a powerful regulator of Blood pressure. These drugs block Renin-Angiotensin System (RAS, enzyme) which is secretly produced in Kidney. Such few antihypertension drugs were described in Figure 1.
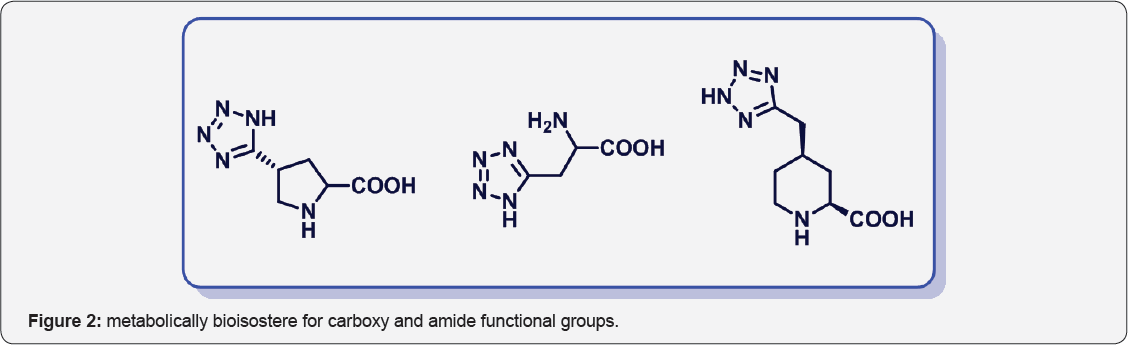
Tetrazole ring was widely used as a metabolically stable bioisostere for carboxy and amide functional groups in molecular design and synthesis of modified amino acids. Such analogs are described in Figure 2.
The generations of Cephalosporin Antibiotics played a vital role in diagnosis process. Huge research work has been done for development of these drug analogs shown in Figure 3.


Dave, C.G shah and coworkers synthesized 7, 9-Disubstituted- 7H-tetrazolo[1,5-c] pyrrolo[3,2-e] pyrimidines and evaluated their biological activity. Below compound exhibited better activity than ampicillin against all tested culture.
Venkataraman Subramanian and co worker developed a novel route for terazole analgous of Cl-Amidines and F- Amidines.
As a part of our research work, we synthesized a high potential tetrazolidine analogous were synthesized as a urea and carbamates derivatives using conventional methods of cycloaddition reactions and curtius rearrangement reactions. Present method we avoid toxic and hazard reagents during synthesis of urea and carbamate derivatives.
Material and Methods
All Amines and alcohols and Reagents collected from commercial sources (Aldrich, Alfa Aesar). THF and Toluene were thoroughly dried using sodum metal and benzophenone before conduct reaction. DMF was dried using CaH. Alcohols (EtOH, MeOH) were super dried using Grignard method (Mg, I2). The Curtius rearrangement reactions were conducted in sealed tube. These derivatives were characterized by using Analytical methods like IR, NMR (400 m Hz, Bruker). The melting points were recorded using on a WRS-1A Digital Melting Point Apparatus without correction. Infrared spectra were taken using an AVATAR 370 FT-IR spectrometer. 1HNMR, 13CNMR spectra were recorded with a Bruker spectrometer operating at 400MHz used as a trimethylsilane as a reference and values were recorded in ppm. The progress of reaction was monitored using TLC system and I2 spray and KMnO4 TLC strain. The crude compounds were purified using column chromatography (100-200 mesh silica) and Combi-flash chromatography. The hydrogenation process was carried out using parr shaker.
Objective Of This Research
Present work is corresponding to develop novel synthetic route for preparation of the quinoline attached tetrazolidine urea and N-carbamate derivatives and characterized by IR and 1HNMR.
Experimental Methods
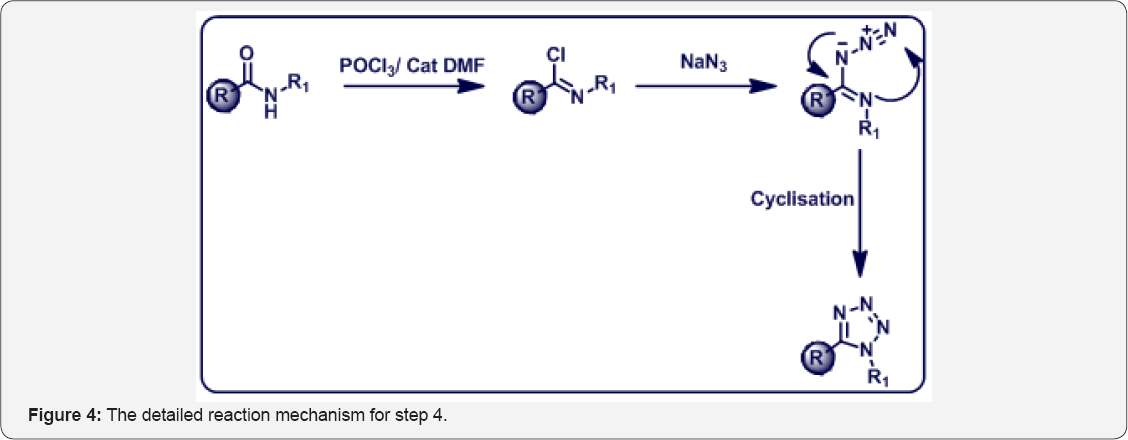
In this research work, we prepared below compounds and mentioned in step wise manner. The detailed scheme was given in Scheme 1. The Reaction mechanism for step 4 was mentioned in Figure 4.
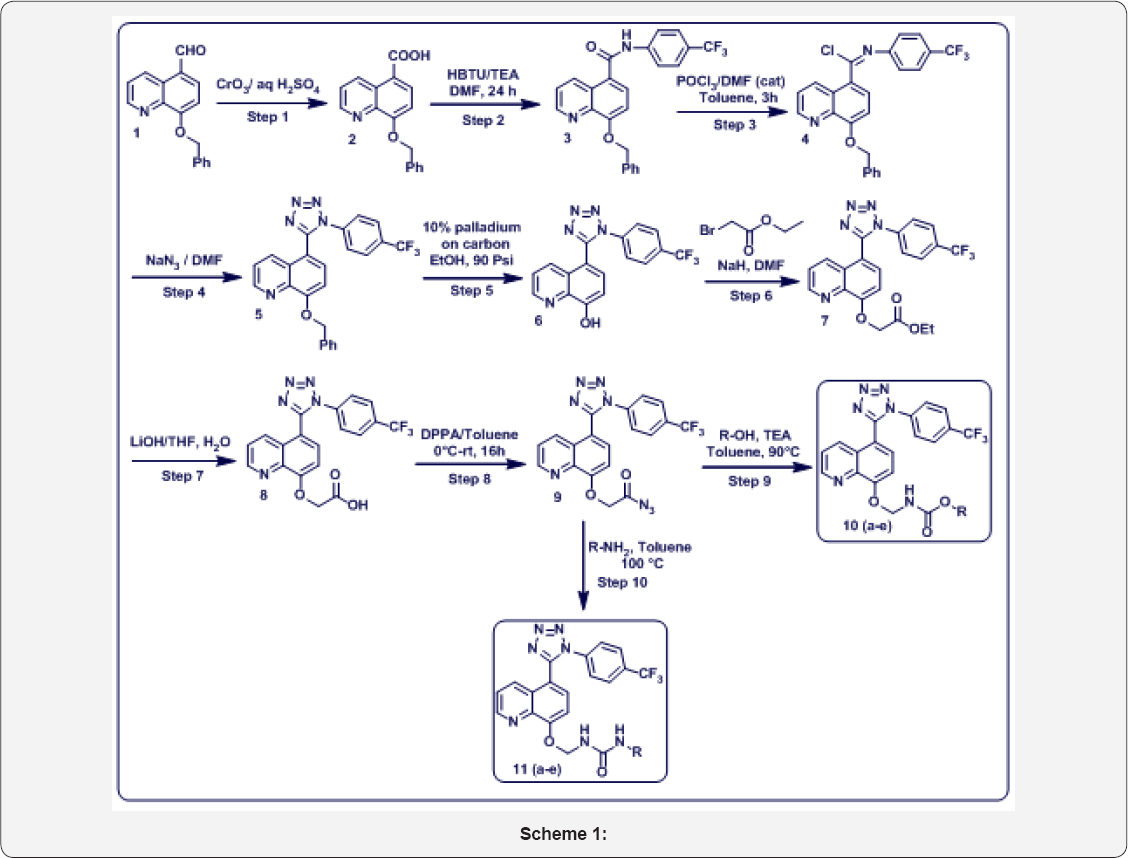
a) Step-1: 8-(benzyloxy) quinoline-5-carboxylic acid (2).
b) Step-2: 8-(benzyloxy)-N-(4-(trifluoromethyl) phenyl) quinoline-5-carboxamide (3).
c) Step-3: (E)-8-(benzyloxy)-N-(4-(trifluoromethyl) phenyl) quinoline-5-carbimidoyl chloride (4).
d) Step-4: 8-(benzyloxy)-5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5-yl) quinoline (5).
e) Step-5: 5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol- 5-yl) quinolin-8-ol (6).
f) Step-6: Ethyl 2-((5-(1-(4-(trifluoromethyl) phenyl)- 1H-tetrazol-5-yl) quinolin-8-yl) oxy) acetate (7).
g) Step-7: 2-((5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinolin-8-yl) oxy) acetic acid (8).
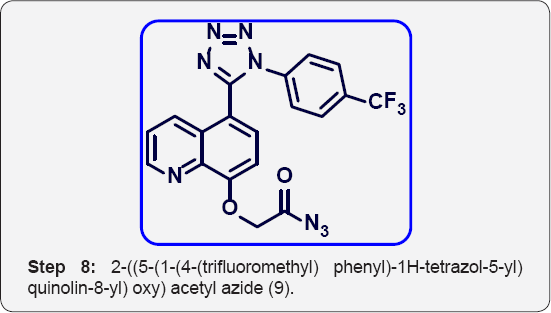
h) Step-8: 2-((5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinolin-8-yl) oxy) acetyl azide (9).
i) Step-9: Substituted (((5-(1-(4-(trifluoromethyl) phenyl)- 1H-tetrazol-5-yl) quinolin-8 yl) oxy) methyl) carbamate (10 a-e).
j) Step-10: substituted-3- (((5- (1- (4- (trifluoromethyl) phenyl)-1H-tetrazol-5-yl) quinolin-8-yl) oxy) methyl) urea 11 a-e).
Scheme 1
Reaction Conditions:
a) Step 1: CrO3/H2SO4, 0 °C, 3 h.
b) Step 2: HBTU /DIPEA (3 eq), DMF. Rt, 16 h.
c) Step 3: POCl3/DMF (cat), 0°C-50°C, 3 h.
d) Step 4: NaN3 / DMF, 80 °C, 4 h.
e) Step 5: 10% Palladium on Carbon, H2/ 90 psi.
f) Step 6: Ethyl Bromo Acetate, NaH, 0°C- RT, 6 h.
g) Step 7: LiOH, THF/H2O (1: 4), 16 h.
h) Step 8: DPPA / Toluene, 0°C, 8 h.
i) Step 9: R-OH, Toluene, 90 °C, 5 h, and sealed tube.
Reaction mechanism for Step 2:
Step 1:8-(benzyloxy) quinoline-5-carboxylic acid (2):
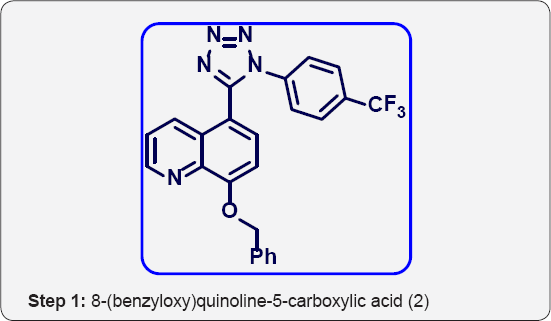
Zones Reagent: In a 1 lit 3 neck round bottom flask fitted with mechanical stirrer, CrO3 (28 g, 0.285 mol) was dissolve in water (50 mL) and cooled to 0 °C for 10 min. Then added H2SO4 (5.5M, 130 mL, 0.3 eq) drop wise for 30 min at -5°C. The reaction mixture was stirred for 10 min. In Another RBF 8-(benzyloxy) quinoline-5-carbaldehyde (25 g, 0.09 mol) was dissolve in Acetone (250 mL) and cooled to 0°C. The above reagent (Zones reagent) was added drop wise for 1 h and stirred for 2 h. The progress of reaction was monitored by TLC. After completion, Reaction mixture was poured in ice cold water and stirred for 30 min. The reaction mixture was extracted with EtOAc (3 x 250 mL). The reaction mixture was filtered on cellite bed. The organic layer were separated and dried over anhydrous Na2SO4, filtered and evaporated under vacuum to give black crude product. The crude was purified by column chromatography (100-200 mesh silica, Eluent: Pure EtOAc) isolated 8-(benzyloxy) quinoline- 5-carboxylic acid (15 g, Yield: 57%) as a white solid. MP. 282285 °C. IR (KBr, cm-1): 3400, 3010, 1710, 1580, 1440, 770, 655. !HNMR (d6-DMSO, 400 mHz): 4.9 (s, 1H), 7.35-7.5 (m, 5H), 7.8 (t, 1H), 8.4 (d, 1H), 8.9 (d, 1H), 9.5 (d, 1H), 10.2 (brs, 1H).
Step 2: 8-(benzyloxy)-N-(4-(trifluoromethyl) phenyl) quinoline-5-carboxamide (3):
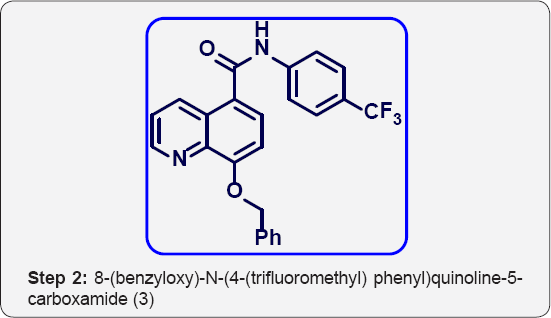
To a mixture of 8-(benzyloxy)quinoline-5-carboxylic acid (15g, 0.053 mol), 4-(trifluoromethyl) aniline (8.6 g, 0.053 mol) in DMF (100 mL ) was added Di-isopropyl ethyl amine (DIPEA) (25 mL, 0.159 mol) and cooled to 0 °C. Then HBTU (22 g, 0.06mol) was added and stirred at room temperature for 16 h. The progress of reaction was monitored by TLC and iodine strain. After completion, reaction mixture was poured in ice cold water (300 mL) and extracted with Et OAc (3 x 300 mL).
The organic layer was separated and washed with brine solution (100 mL). The organic layer was collected and dried over anhydrous Na2SO4, filtered and evaporated under vacuum to give crude residue. The crude product was purified by Combi- flash column chromatography (230-400 meshsilica, Eluent: 10% MeOH-CHCl3) isolated 8-(benzyloxy)- N -(4-(trifluoromethyl) phenyl) quinoline-5-carboxamide (3) (16 g, yield: 72 %) as a white solid. M.p: 352-355 °C. IR (KBr, cm-1): 3410, 3020, 1700, 1610, 1320, 1420, 780, 685. 1HNMR (d6-DMSO, 400 mHz): 5.1 (s, 2H), 7.2 (d, 1H), 7.38-7.5 (m, 5H), 7.6 (d, 2H), 7.63 (d, 2H), 7.83 (t, 1H), 8.8 (br , 2H), 9.5 (d, 1H).
Step 3: (E)-8-(benzyloxy)-N-(4-(trifluoromethyl) phenyl) quinoline-5-carbimidoyl chloride (4):
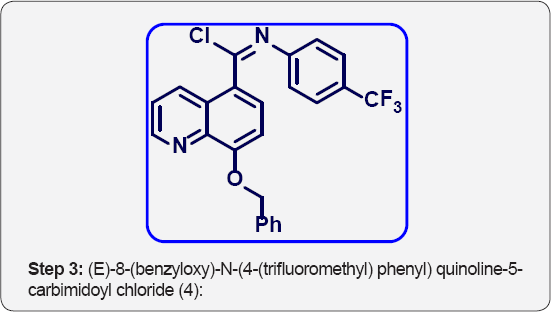
8-(benzyloxy)-N-(4-(trifluoromethyl) phenyl) quinoline- 5-carboxamide (3) (15 g, 0.035 mol) in POCl3 (150 mL) was cooled to 0 °C. Then added DMF (cat, 1mL) drop wise and stirred at room temperature for 1h. The reaction mixture was heated at 60 °C for 3h. The progress of reaction was monitored by TLC. After completion, reaction mixture was cooled to room temperature. The POCl3 was evaporated under high vacuum to give crude residue. The residue was co-distilled with dry toluene (2 x 100 ml) to give crude product. The crude was carried to next step without further purification. This compound data was not analyzed.
Step 4: 8-(benzyloxy)-5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinoline (5):
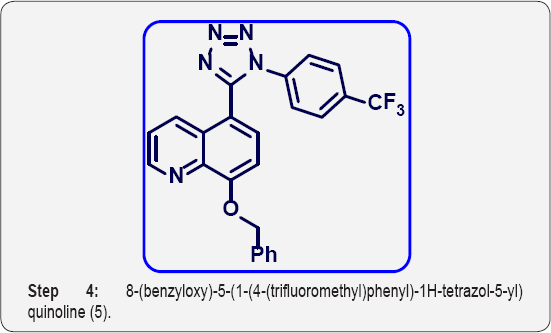
(E)-8-(benzyloxy)-N-(4-(trifluoromethyl) phenyl) quinoline-5- carbimidoyl chloride (4) (15g, 0.035 mol) in dry DMF (70 mL) was cooled to 0°C. Then sodium azide (3 eq) was added portionwise and stirred at room temperature for 1 h. After that, Reaction mixture was heated at 80 °C for 4 h. The progress of reaction was monitored by TLC. After completion, reaction mixture was cooled to 0 °C and poured in ice cold water (200 mL) and basified up to PH-8 with sat aq NaHCO3 sol.
The aqueous layer was extracted with EtOAc (3 x 100 mL). The organic layer were collected and dried over anhydrous Na2SO4, filtered and evaporated under vacuum to give crude product. The crude was purified by column chromatography (100-200 mesh silica, Eluent: 50% EtOAc-Pet Ether) isolated 8-(benzyloxy)-5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5- yl) quinoline (5) (8g, Yieild : 50%) as a pale yellow solid. M.p: 280-282 °C. IR (KBr, cm-1): 3010, 1550, 1510, 820, 655. 1HNMR (d6-DMSO, 400 mHz): 5.10 (s, 2H), 7.1 (d, 1H), 7.4-7.68 (m, 11H),8.4 (d, 1H), 8.78 (d, 1H).
Step 5: 5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5-yl) quinolin-8-ol (5):
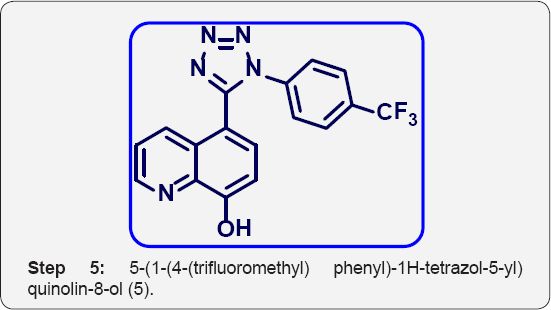
8-(benzyloxy)-5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5- yl) quinoline (5) (8 g, 0.012 mol) in MeOH (100 mL) was added 10% palladium on carbon and TFA (10 mL) and hydrogenated at 80 Psi using parr shaker for 5 h at room temperature. The progress ofreaction was monitored by TLC. After completion, Reaction mixture was filtered on cellite bed and washed with MeOH (2 x 50 mL). The organic layer was collected and evaporated under vacuum to give crude residue. The residue was purified by combi-flash column chromatography isolated 5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5-yl) quinolin- 8-ol (6) (5.5 g, Yield: 88%). as a white solid.M.p:310-315 °C. IR (KBr, cm-1): 3300, 3040, 1580, 1425, 1150,760, 691, 1HNMR (d6-DMSO, 400 mHz): 7.2 (d, 1H), 7.5-7.62 (m, 5H), 7.8 (d, 1H), 8.4(d, 1H), 8.88 (d, 1H).10.1(brs, 1H).
Step 6: Ethyl 2-((5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinolin-8-yl) oxy) acetate (7):
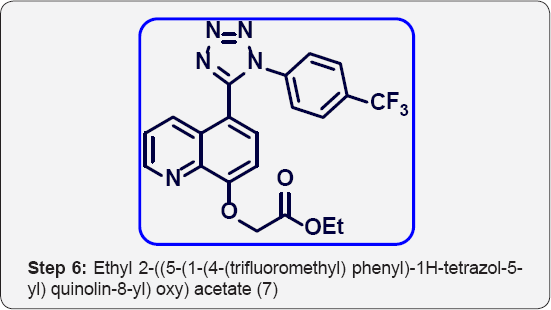
To a solution 5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5- yl) quinolin-8-ol (6) (5 g, 0.014 mol), in DMF (50 mL) was cooled to 0°C. Then added sodium hydride (1.7 g, 0.042 mol, 3 eq) portion wise and stirred for 30 min. To that Ethyl bromo acetate (1.71 mL, 0.0154 mol) was added drop wise and stirred for 6 h at room temperature. The progress of reaction was monitored by TLC. After completion, reaction mixture was poured in ice cold water (100 mL) and basified with aq Na2CO3 up to PH-8 and extracted with EtOAc (2 x 100 mL). The organic layer were separated and washed with brine (25 mL), and dried over anhydrous Na2SO4, filtered and evaporated under vacuum to give give crude product. The crude was purified by column chromatography (100-200 mesh silica, Eluent: 60% EtOAc- Pet ether) isolated ethyl 2-((5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinolin-8-yl) oxy) acetate (4 g, Yield: 64%) as a semi brown colour liquid. M.p: 255-258 °C. IR (KBr, cm-1): 3040, 1640, 1560, 1440, 1130, 780, 698. 1HNMR (d6-DMSO, 400 mHz): 1.4 (t, 3H), 3.8 (q, 2H), 4.8 (s, 2H), 7.23 (d, 1H), 7.5-7.62 (m, 5H), 7.75 (d, 1H), 8.39 (d, 1H), 8.7(d, 1H).
Step 7: 2-((5-(1-(4-(trifluoromethyl)phenyl)-1H-tetrazol-5- yl)quinolin-8-yl)oxy)acetic acid (8):
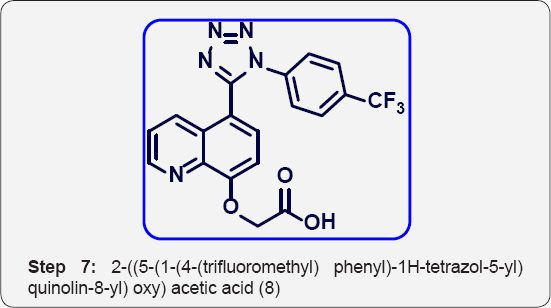
Ethyl2-((5-(1-(4-(trifluoromethyl)phenyl)-1H-tetrazol-5- yl) quinolin-8-yl)oxy)acetate (8) (4 g, 0.009 mol) in THF/H2O (40 mL/10mL, 4:1) was added LiOH (4 eq) and stirred at room temperature for 16 h. The progress of reaction was monitored by TLC. After completion, reaction mixture was poured in ice cold water (100 mL) and acidified with aq NaHSO3 (100 mL ) up to PH-4 and extracted with EtOAc (3 x 00 mL). The organic layer were collected and dried over anhydrous Na2SO4, filtered and evaporated under vacuum to give crude product. The crude was purified by column chromatography (100-200 mesh silica, Eluent: 80% EtOAc- Pet ether) isolated 2-((5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5-yl) quinolin-8-yl) oxy) acetic acid (3.5, Yield: 93%) as a white solid. M.p: 285-288 °C. IR (KBr, cm-1): 3500, 3030, 1690, 1560, 1440, 760, 610, 1HNMR (d6- DMSO, 400 mHz): 4.8 (s, 2H), 7.3 (d, 1H), 7.5-7.65 (m, 5H), 7.78 (d, 1H), 8.38 (d, 1H), 8.8(d, 1H), 10.4 (brs, 1H).
Step 8: 2-((5-(1-(4-(trifluoromethyl) phenyl) -1H-tetrazol-5-yl) quinolin-8-yl) oxy) acetyl azide (9):
To a solution of 2-((5-(1-(4-(trifluoromethyl)phenyl)-1H- tetrazol-5-yl)quinolin-8-yl)oxy) acetic acid (3.5 g, 0.008 mol) in toluene (35 mL) was added DPPA (2.25 mL, 0.014 mol) at 0°C and stirred at room temperature for 8 h. The progress of reaction was monitored by TLC. After completion, reaction mixture was evaporated under vacuum to give crude residue.The crude residue was co-distilled with Toluene (2x30 mL) isolated 2-((5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5- yl) quinolin-8-yl) oxy) acetyl azide (4.5 g) as a liquid. The crude residue was carried to next step without further purification.
Step 9: Substituted (((5-(1-(4-(trifluoromethyl)phenyl)- 1H-tetrazol-5-yl) quinolin-8-yl)oxy)methyl) carbamate (10):
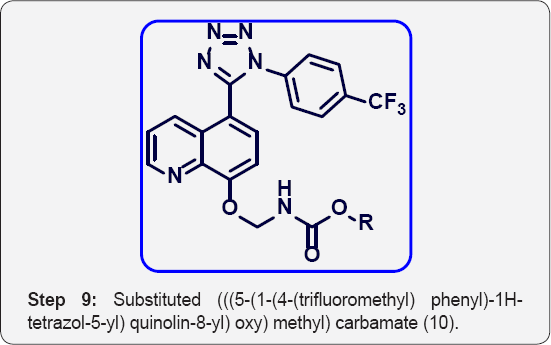
To a solution of 2-((5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinolin-8-yl) oxy) acetyl azide (9) (250 mg,0. 568 mmol) in Dry toluene (10 mL) was added TEA (1.7 mmol, 3 eq), Alcohol (1.1 eq) and heated at 100 °C for 5 h in sealed tube (50 mL). The progress of reaction was monitored by TLC. After completion, reaction was evaporated under vacuum to give crude product. The crude was purified by Combi-flash column chromatography (100-200 mesh silica, Eluent: 70% EtOAc-Pet ether), isolated substituted (((5-(1-(4-(trifluoromethyl) phenyl)- 1H-tetrazol-5-yl)quino lin-8-yl)oxy)methyl)carbamate10 (a-e). The list of carbonate derivatives was given in Table 1.
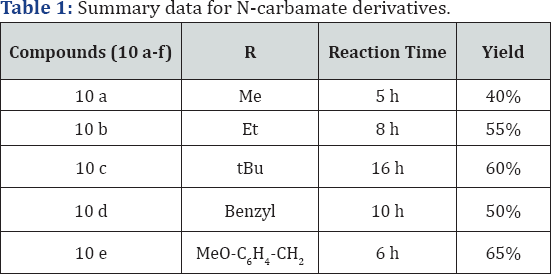
Methyl (((5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol- 5-yl) quinolin-8-yl) oxy) methyl) carbamate (10a): (Figure 6) M.p: 280-283 °C. IR (KBr, cm-1): 3450, 3030, 1645, 1350,780, 645, 1HNMR (d6-DMSO, 400 mHz): 3.5(s, 2H), 5.5(s, 2H), 7.2 (d, 1H), 7.5-7.64 (m, 5H), 8.1 (m, 3H), 8.4(d, 1H), 8.8(d, 1H). 13CNMR (d6-DMSO, 400 mHz) : 51, 75, 108, 122, 123, 124.5, 125,131,132, 135, 139, 149, 155, 157, 163.
Ethyl (((5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol- 5-yl) quinolin-8-yl) oxy) methyl) carbamate (10b): (Figure 7) M.p: 290-293 °C. IR (KBr, cm-1): 3450, 3035, 1650, 1350,785, 650, 1HNMR (d6-DMSO, 400 mHz): 1.25 (t, 3H), 4.2 (q, 2H), 5.4 (s, 2H), 7.2 (d, 1H) 7.55-7.63 (m, 5H), 7.9 (m, 2H), 8.38 (d, 1H), 8.85 (d, 1H). 13CNMR (d6-DMSO, 400 MHz): 15, 62, 77, 107.5, 122, 123, 124, 125, 131, 132, 135, 140, 150, 155, 156, and 164.
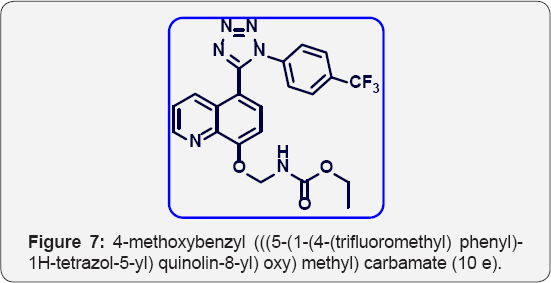
Tert-butyl (((5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinolin-8-yl) oxy) methyl) carbamate (10c):(Figure 8) M.P.: 250-253 °C, IR (KBr, cm-1):.3470, 3040, 1670, 1380,770, 640. 1HNMR (d6-DMSO, 400 mHz): 1.5 (s, 9H), 5.5 (s, 2H), 7.18 (d, 1H) 7.55-7.63 (m, 5H), 7.8 (m, 2H), 8.37 (d, 1H), 8.83(d, 1H). 13CNMR (d6-DMSO, 400 mHz): 30, 76, 79, 107, 121, 122.8, 124.9, 131, 132, 136, 139, 148, 155, 156, 163.8.
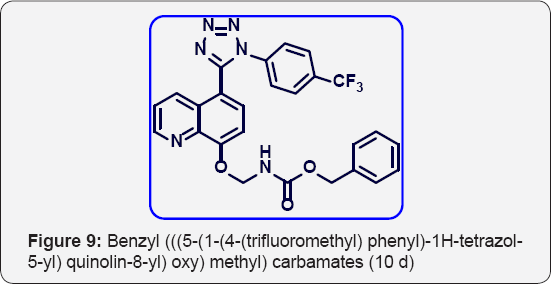
Benzyl (((5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol- 5-yl) quinolin-8-yl) oxy) methyl) carbamate (10d): (Figure 9) M.p: 300-303 °C. IR (KBr, cm-1): 3490, 3030, 1650, 1380,780, 660. 1HNMR (d6-DMSO, 400 mHz): 5.1 (s, 2H), 5.6 (s, 2H), 7.2 (d, 1H) 7.55-7.63 (m, 10 H), 7.85 (m, 2H), 8.39 (d, 1H), 8.85(d, 1H). 13CNMR (d6-DMSO, 400 mHz): 68, 78, 108, 122, 123, 124, 124.5, 125.3, 127, 127.6, 129,131,132,135.2,136, 139, 148, 155,156,163.

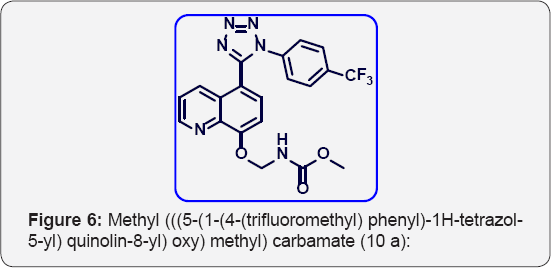
4-methoxybenzyl (((5-(1-(4-(trifluoromethyl) phenyl) -1H-tetrazol-5-yl) quinolin-8yl) oxy) methyl) carbamates (10e). (Figure 10) M.P.320-323 °C. IR (KBr, cm-1): 3460, 3050, 1640, 1370,785, 665. 1HNMR (d6-DMSO, 400 mHz): 3.83 (s, 3H), 4.9 (s, 2H), 5.65 (s, 2H), 6.9-7.1 (m, 5H), 7.55-7.62 (m, 5 H), 7.85 (m, 2H), 8.38 (d, 1H), 8.87 (d, 1H). 13CNMR (d6-DMSO, 400 mHz): 56, 67, 76, 108, 115, 122, 123, 123.8, 124, 124.1, 125.3, 128, 129,130, 131.9, 132, 135.2, 139, 149, 154, 156, 158, 164.
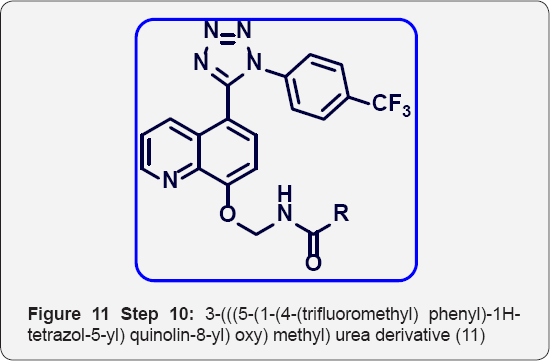
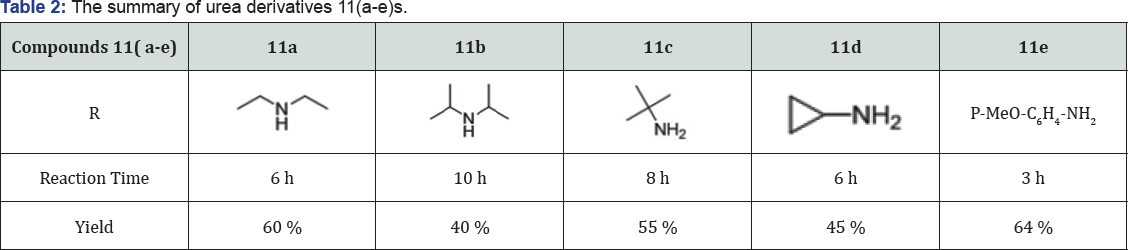
Step 10: 3-(((5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinolin-8-yl) oxy) methyl) urea derivative (11) : (Figure 11) To a solution of 2-((5-(1-(4-(trifluoromethyl) phenyl)-1H-tetrazol-5-yl) quinolin-8-yl) oxy) acetyl azide (9) (250 mg, 0.568 mmol) in Dry toluene (10 mL) was added Amine (1.3 eq) and heated at 100 °C for 5 h in sealed tube (50 mL). The progress of reaction was monitored by TLC. After completion, reaction was evaporated under vacuum to give crude product. The crude was purified by Combi-flash column chromatography (100-200 mesh silica, Eluent: (5-10 % MeOH-CHCl3), isolated 1-(((5-(1-(4-(trifluoromethyl)phenyl)-1H-tetrazol-5-yl) quinolin-8-yl) oxy) methyl) urea derivatives 11 (a-e). The list of urea derivatives was mentioned in Table 2.
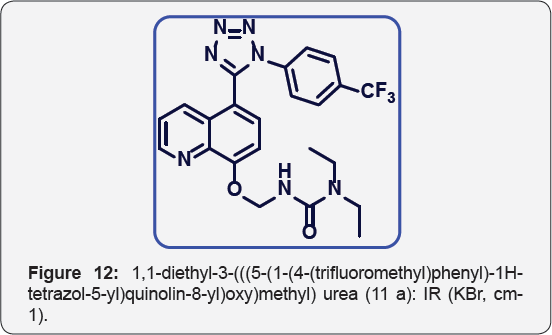
1, 1-diethyl-3-(((5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinolin-8-yl) oxy) methyl) urea (11a): IR (KBr, cm-1): (Figure 12) White solid.lR (KBr, cm-1): 3410, 3010, 1730, 1655, 1450, 1320,780, 650. 1HNMR (d6-DMSO, 400 mHz):
1.5 (t, 6H), 3.8 (q, 4H), 5.4 (s, 2H), 7.15 (d, 1H), 7.5-7.6 (m, 5H),
7.8 (m, 2H), 8.38 (d, 1H), 8.8(d, 1H). 13CNMR (d6-DMSO, 400 mHz): 15, 45, 78, 108, 122, 123, 124, 124.5, 125, 131, 132, 135, 139, 149,155,157, 164.
1-diisopropyl-3-(((5-(1-(4-(trifluoromethyl) phenyl)- 1H-tetrazol-5-yl) quinolin-8-yl) oxy) methyl) urea (11b): IR(KBr, cm-1): (Figure 13) off white colour solid. IR (KBr, cm-1): 3430, 3020, 1720, 1645, 1440, 1330,760, 640. 1HNMR (d6-DMSO, 400 mHz): 1.4 (d, 12H), 3.8 (q, 2H), 5.6 (s, 2H), 7.1 (d, 1H), 7.57.6 (m, 5H), 7.8 (m, 2H), 8.4 (d, 1H), 8.83(d, 1H). 13CNMR (d6- DMSO, 400 mHz): 21, 55, 77, 107, 121, 123, 124, 124.5, 131, 132, 133, 135, 139, 148, 154, 158, 165.

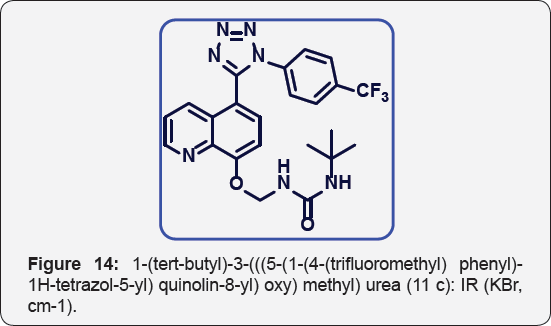
1-(tert-butyl)-3-(((5-(1-(4-(trifluoromethyl) phenyl)-1H- tetrazol-5-yl) quinolin-8-yl) oxy) methyl) urea (11c): IR(KBr, cm-1): (Figure 14) White colour solid. IR (KBr, cm-1): 3460, 3040, 1650, 1460, 1380, 680, 620. 1HNMR (d6-DMSO, 400 mHz):
1.5 (s, 9H), 5.68 (s, 2H), 7.15 (d, 1H), 7.5-7.6 (m, 5H), 7.8 (m, 2H), 8.1 (brs, 1H), 8.43 (d, 1H), 8.84(d, 1H). 13CNMR (d6-DMSO, 400 mHz): 30, 58, 76, 108, 122, 123, 124, 124.5, 131.8, 13 2, 133, 135, 139, 146, 155, 157, 164.
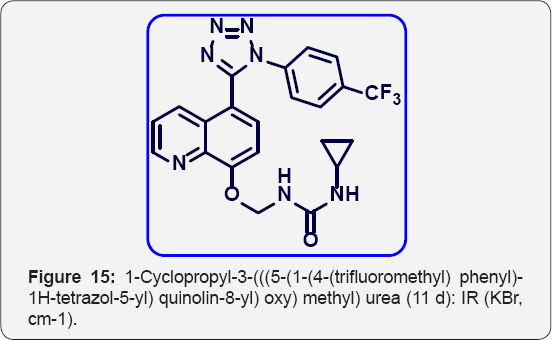
1-Cyclopropyl-3-(((5-(1-(4-(trifluoromethyl) phenyl)- 1H-tetrazol-5-yl) quinolin-8-yl) oxy) methyl)urea (11 d): IR (KBr, cm-1): (Figure 15) Pale yellow colour solid. IR (KBr, cm- 1): 3510, 3430, 3040, 1650, 1460, 1340, 690, 550. 1HNMR ( d6- DMSO, 400 mHz) : 0.9 (t, 2H), 1.1 (t, 2H), 2.5 (m, 1H), 5.7 (s, 2H),3.8 (brs, 1H), 7.15 (d, 1H), 7.5-7.6 (m, 5H), 7.81 (m, 2H), 8.3 (brs, 1H), 8.4 (d, 1H), 8.80(d, 1H). 13CNMR (d6-DMSO, 400 mHz): 8, 30, 76, 108, 122, 123.1, 123.5, 124, 125.3, 130.1, 132, 135, 139.1, 149, 155, 157, 164.
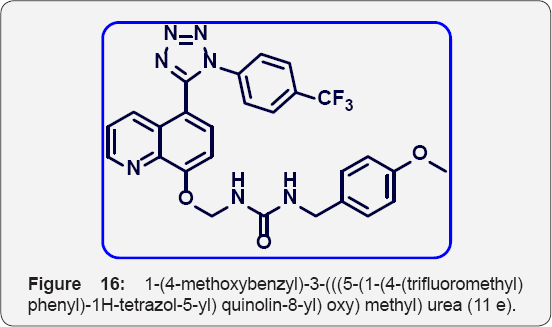
1-(4-methoxybenzyl)-3-(((5-(1-(4-(trifluoromethyl) phenyl) -1H-tetrazol-5-yl) quinolin-8-yl) oxy) methyl) urea (11e): (Figure 16) White colour solid IR (KBr, cm-1): 3540, 3430, 3030, 1640, 1440, 1350, 730. 1HNMR ( d6-DMSO, 400 mHz] : 3.6 (s, 3H), 4.2 (s, 2H), 5.7 (s, 2H), 6.8 (d, 2H), 7.1 (d, 1H), 7.25 (d, 2H), 7.5-7.6 2(m, 5H), 7.83 (m, 2H), 8.25 (brs, 1H), 8.41 (d, 1H), 8.84(d, 1H). 13CNMR (d6-DMSO, 400 mHz): 45, 56, 77, 107.5, 115, 121, 122, 123, 124, 130, 130.5, 130.8, 132.2, 135, 140, 149, 155, 157, 158, 163.
Conclusion
Present research work we have developed a novel route for synthesis of high potential pharmacological carbamate and urea derivatives using conventional and scalable route. We avoid by products in curtius rearrangement by using dry toluene and reactions were executed under argon atmosphere. We avoid hazard reagents like (phosgene and acid chloride methods) for preparation of urea and carbamates derivatives. We employed very easiest method for preparation of urea and carbamates derivatives and avoid side products. We are planning to these derivatives check for biological evolution. The biological evolution details will include next journal [13-20].
Acknowledgement
I sincerely thankful to my guide (Dr L.K Ravindranath), co-workers, Department of Chemistry, Sri Krishnadevaraya University, (Anantapur, Andhra Pradesh, India ) and Gvk Bio sciences for providing laboratory and analytical facilities.
References
- Vijaya Bhaskar Vangala, Rama Mohan Hindupur, Hari Narayan Pati (2014) A Review on Synthesis of Antihypertensive Sartan Drugs International Journal of Pharma Research & Review 3(11):46-56.
- Maruthamuthu, Shameela Rajam, Christina Ruby Stella P, Bharathi Dileepan AG, et al. (2016) The chemistry and biological significance of imidazole, benzimidazole, benzoxazole, tetrazole and quinazolinone nucleus. Journal of Chemical and Pharmaceutical Research 8(5): 505526.
- M Rajendran, D Devapiriam (2015) DFT calculations for corrosion inhibition of copper by tetrazole derivatives. Journal of Chemical and Pharmaceutical Research 7(1): 763-773.
- M Maria Dorathi Anu, M Jayanthi, S Damodar Kumar, S Raja (2013) Synthesis Characterization, Antibacterial & Anti-Inflammatory Effects Of Substituted Tetrazole Derivatives Based On Different Types Of Carbazone And Benzaldehyde. International Journal of Chem Tech Research Vol.5, No.4, pp 1982-1990.
- VH Bhaskar, PB Mohite (2010) Synthesis, Characterization and Evaluation Of Anticancer Activity Of Some Tetrazole Derivatives. Journal of Optoelectronics and Biomedical Materials 2(4): 249-259.
- MA Kale, MR Peharkar (2013) Synthesis Of Some Novel Tetrazole Substituted Benzimidazoles And Their Evaluation As Antioxidants. International Journal of Pharma and Bio Sciences 4(4): 675-681.
- Davood Habibi, Hiva Nabavi, and Mahmoud Nasrollahzadeh (2013) Silica Sulfuric Acid as an Efficient Heterogeneous Catalyst for the Solvent-Free Synthesis of 1-Substituted 1H-1,2,3,4-Tetrazoles. Journal of Chemistry.
- Cheng-Xi Wei, Ming Bian, Guo-Hua Gong (2015) Tetrazolium Compounds: Synthesis and Applications in Medicine. Molecules 20(4): 5528-5553.
- Fatemeh Darvish, Shima Khazraee (2015) FeCl3 Catalyzed One Pot Synthesis of 1-Substituted 1H-1,2,3,4-Tetrazoles under Solvent-Free Conditions. International Journal of Organic Chemistry 5: 75-80.
- Venkataraman Subramanian, Jason S Knight, Sangram Parelkar, Lynne Anguish, et al. (2015) Design, Synthesis, and Biological Evaluation of Tetrazole Analogs of Cl-Amidine as Protein Arginine Deiminase Inhibitors. Journal of Medicinal chemistry 58: 1337-1344.
- VA Ostrovskii, RE Trifonov, EA Popova (2012) Medicinal chemistry of tetrazoles. Russian Chemical Bulletin 61(4): 768-780.
- Mohite PB, Bhaskar VH (2011) Potential Pharmacological Activities of Tetrazoles in The New Millennium. international Journal of Pharm Tech Research 3(3): 1557-1566.
- Ivanildo Mangueira da Silva, Joao da Silva Filho (2014) Synthesis and Antimicrobial Activities of 5-Arylidene-thiazolidine-2,4-dione Derivatives. Med Research international Volume 8.
- H Sharghia, R Khalifeha b, F Moeinia, MH Beyzavia, et al. (2011) Mannich reaction of secondary amines, aldehydes and alkynes in water using Cu/C nanoparticles as a heterogeneous catalyst. J Iran Chem Soc 8: S89-S103.
- Archana Kapoor, Neha Khare, Der Pharmacia Lettre (2016) Synthesis and evaluation of 2-aryl substituted benzimidazole derivatives bearing 1,3,4-oxadiazole nucleus for antimicrobial activity. 8 (12):143-148.
- Andreea-Teodora Panzariu, Maria Aprotosoaei, Lenuta profire (2013) Synthesis Of New Thiazolidine-2,4-Dione Derivatives And Their Antimicrobial And Antitubercular Activity. Indian jounal of pharmacy and Biotecnology (1): P 496.
- Devprakash, Udaykumar A Bhoi (2011) A complete review of thiazolidine-4-ones. Journal of Pharmacy Research 4(7), 2436-2440.
- BA Bhaviskar, SS Khadabadi, SL Deore (2013) Synthesis and Evaluation of Some New Thiazolidin-4-One Derivatives as Potential Antimicrobial Agents. Journal of Chemistry: p. 6.
- Tribhuvan Singh, Deepak Khobragade (2014) Synthesis and Evaluation of Thiazolidine-4-One for their Antibacterial Activity. JPSBR 4(1): 110113.
- Kishan D Patel, Chhaganbhai N Patel, Grishma M Patel (2016) Microwave Assisted Synthesis and Antidiabetic Activity of Novel 5-[4-(Substituted) Benzylidine]Thiazolidine-2,4-Dione. Med Chem (Los Angeles) 6: 10.






























