Synthesis, X-Ray diffraction, Trypanocidal activity and Molecular Studies of Benzoyl Phthalimides and Isoquinolones
Paola L Ramirez-Hernandez1, Erick Suarez-Contreras2, Benjamin Nogueda-Torres2, Marco A. Leyva-Ramirez3, Efren V Garcia-Baez1 and Marco Brito-Arias1*
1Unidad Profesional Interdisciplinaria de Biotecnologfa, IPN, Mexico
2Escuela Nacional de Ciencia Biologicas, Carpio y Plan de Ayala Colonia Santo Tomas, Mexico
3Av.Instituto Politecnico Nacional 2508, San Pedro Zacatenco, Mexico
Submission: August 05, 2017; Published: August 23, 2017
*Corresponding author: Marco Brito-Arias, Unidad Profesional Interdisciplinaria de Biotecnologfa, IPN, Avenida Acueducto s/n Barrio la Laguna Ticoman, 07340 Mexico, DF, Mexico, Email: mbrito@ipn.mx
How to cite this article: P L Ramírez-Hernandez, E Suárez-Contreras, B Nogueda-Torres, M A Leyva-Ramírez, et al. Synthesis, X-Ray diffraction, Trypanocidal activity and Molecular Studies of Benzoyl Phthalimides and Isoquinolones. Organic & Medicinal Chem IJ. 2017; 3(2): 555608. DOI: 10.19080/ OMCIJ.2017.03.555608.
Abstract
α-Phthalimide acetophenones 1a-b, 3-benzoyl isoquinolones 2a-b were synthesized by following two pathways and evaluated as trypanosoal inhibitors in vitro, showing weak activity for 1a-b and 2a, and significant for 2b as compared with the reference drugs benznidazol. Also docking studies were carried out to determine the binding mode, the scoring functions and the interactions with the residues at the active site of the cruzain target enzyme.
Keywords: Benzoyl isoquinolones; Trypanosoma cruzi; Molecular modeling; Phtalimide ketones; Cruzain inhibition
Abbreviations:Farnesyl Pyrophosphate Synthase (FPPS); Trans-Sialidase (TS); Trypanothione Reductase (TR); Glucose 6-Phosphate- Dehydrogenase (GPDH); Glyceraldehyde 3-Phosphate-Dehydrogenase (GAPDH); Hydroxyacid Dehydrogenase (HDH); Dimethyl Sulphoxide (DMSO)
Introduction
The development of improved drugs against parasitic diseases continues to be of high priority in some countries due their high incidence, accompanied by high toxicity treatments and therapeutic mixed results when the infection is beyond the acute and short-term phases. The protozoal infections such as Chagas disease, Leishmaniasis, and Malaria, have a significant impact in countries that are in tropical and subtropical world regions, mainly in Asia, Africa and Latin America. American trypanosomiasis or Chagas disease, caused by the hemoflagelate protozoa Trypanosoma cruzi, is an endemic disease in Latin America and represents a health problem estimated in 16-18 million people infected and there are 25-90 at risk of acquiring the parasite [1,2].
The current treatment against trypanosomiasis includes the nitro imidazole benznidazole, and nitrofuranose nifurtimox which besides the decreasing in the number of parasites, particularly benznidazole also have an effect on the motility of parasites. The therapeutic targets that have been used in the design of new drugs against Tc are: farnesyl pyrophosphate synthase (FPPS), trans-sialidase (TS), trypanothione reductase (TR), glucose 6-phosphate-dehydrogenase (GPDH), glyceraldehyde 3-phosphate-dehydrogenase (GAPDH), and a-hydroxyacid dehydrogenase (HDH) and cruzain cystein protease [1].
The cysteine protease cruzain or cruzipain is an essential T. cruzi enzyme and one of the few validated drug targets for Chagas disease. It has a catalytic domain of 215 amino acid residues and a molecular weight of 22704 KDa. The protease is expressed in all of the stages during the life cycle of the parasite and is essential for the replication of its intracellular form [3]. Although Trypanosomiasis is considered a neglected disease, substantial efforts have been devoted in the search of novel candidates with superior efficiency than benznidazole and nifurtimox particularly during the chronic stage.
The use of computational database such as the online ChEMBL which perform the search based on structural and inhibition information from both functional and target assays provided valuable information for identifying compounds with known activities in T. cruzi. For the 2D portion of the analysis, five molecular fingerprints (Atom Pairs, Radial, MACCS keys, TGD, and piDAPH3) were generated for each structure which allowed us to rank compounds most similar to each other.
The structures with the highest scores for each fingerprint contains heterocyclic rings such as benzimidazole, benzoxazole, cumarine, phtalazine, indole, flavone, isatines, isoxazoles and quinolines [4]. Also there have been reported trypanocidal inhibitors with miscellaneous functionalities such as hydrazones [5,6] thiosemicarbazone and semicarbazone [7,8] purine carbonitriles,[9] and a-keto-based inhibitors[10] with promising results. We have previously reported the significant trypanosidal inhibition of compound 2b and its amino derivative, and in this paper we want to make a comparative analysis of their precursors 1a-b, 2a.
Materials and Methods
All materials were purchased as analytical reagents, chemical solvent were distilled before use, Melting points were recorded on Fisher-Jones melting point apparatus and are uncorrected. 1HNMR spectra were recorded at 300 MHz on a Varian spectrometer Mercury model using CDCl3 and DMSO-d6 as solvents. Thin layer chromatography was performed on pre-coated silica gel F254 (Merck Damstadt) with fluorescent indicator.
Chemistry
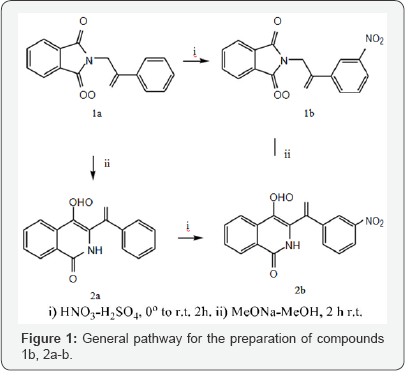
The synthesis of derivatives 1a-b, 2a-b was carried out according to scheme 1 starting from condensation reaction between potassium phtalimide and 2-bromacetophenone in DMF at room temperature to provide 1a which is a common starting material for the preparation of 1b and 2a under nitration and basic conditions respectively. The formation of the isoquinolone nucleus proceeded via the Gabriel-Colman rearrangement [11] as it has been confirmed by x-ray diffraction [12]. Modification of the benzoyl isoquinolone 2a at position 3 was achieved under sulfo-nitric acidic conditions affording a mixture of 2b and 3 in 60:40 % yield (scheme 1 or Figure 1).
Experimental
2-Phtalimide-3'-nitro acetophenone (1b) In a 50 mL round bottom flask were placed compound 1a (1.5 g, 5.65 mmol) and then cooled at 0oC. Slow addition of previously chilled solution of sulfuric acid (5 mL) was added and stirred until complete disolution. A cold mixture of nitric-sulfuric acid (5 mL, 1:1 v/v) was added dropwise and the reaction kept at 0oC during 20 minutes allowing to reach room temperature, maintaining the stirring during further 30 minutes. The reaction mixture was poured in a beaker containing ice to induce precipitation, which was filtered, washed with distilled water (3 x 20 mL), and dried to yield 1.4 g (80 yield) of 1b as a pale brown solid. 1HNMR CDCl3: 5 7.76- 7.80 (m, 3H), 7.93 (dd, 2H), 8.35 (dt, 1H), 8.52 (ddd, 1H), 8.86 (t, 1H). 13CNMR CDCl3: 5 44.5, 123.3, 123.9, 128.5, 130.5, 132.2, 133.9, 134.5, 135.7, 167.9, 189.5. 3(3-Nitrobenzoyl)-4-hydroxi-isoquinolin1-1-one (2b) In a 50 mL round flask containing compound 1b (1g, 3.22 mmol) were cooled at 0oC and then a chilled solution of sulfuric acid (5 mL) were added and stirred until complete dissolution.
A cold mixture of nitric-sulfuric acid (5 mL, 1:1 v/v) were added slowly and the reaction kept at 0oC during 20 minutes allowing to reach room temperature, maintaining the stirring during further 30 minutes. The reaction mixture was poured in a beaker containing ice to induce precipitation, and the resulting solid filtered and washed with distilled water (3 x 20 mL) to give a mixture of 2b which was separated by column chromatography using as elution system hexane-ethyl acetate at different concentration gradients (7:3, 5:5, 3:7) and methanol to produce 0.6 g of 2b 60 % yield as pale yellow solid. 1HNMR of 2b in DMSO-d6: δ 7.72-7.90 (m, 3H), 8.03 (d, 1H, J= 7.2 Hz), 8.11 (d, 1H, J= 6.0 Hz), 8.31 (d, 1H, J= 8.4), 8.43 (d, 1H, J= 9.6), 8.59 (s, 1H), 11.96 (s, 1H). 13CNMR DMSO-d6 δ 124.2, 127.2, 127.7,128.6, 130.9, 131.1, 133.2, 132.9, 134.8, 135.6, 135.9, 148.2, 158.0, 164.0, 166.2, 178.0.
Results and Discussion
X-Ray Difraction of compounds 1b
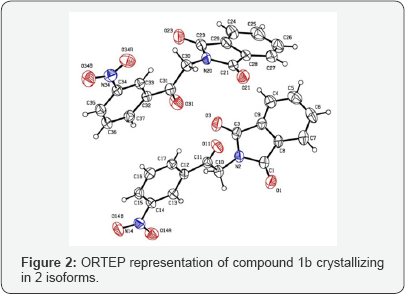
Data on compounds (1b) were collected on Nonius Kappa CCD diffractometer with Mo Kα radiation,[13] at 298 K. The reflections were measured using combined ø and ω scans. The data were reduced by DENZO,[14] the structures solved using SHELXS-97, and refined using SHELXL-97 program package [15]. ORTEP diagram was generated by ORTEP [3,16] the geometric calculations was done by PLATON[16]. All H atoms attached to C and N atoms were fixed geometrically and treated as riding with C-H=0.93Å and N-H=0.86Å with Uiso(H)=1.2Ueq(C,N). Compound (1b) crystallizes with two molecules in the asymmetric unit cell whose parameters are: Molecular Formula (C16H10N2O5): Mr= 310.3, triclinic,P-1, a = 7.9960(8)Å, b = 13.6480(9)Å, c = 14.927l6(8)Å, a = 101.848(5) , α = 105.577(5) , β = 107.115(5), γ = 668.1(3)Å3, Z=4. ORTEP diagrams of the two independent molecules are shown in (Figure 2). As a whole the molecule is non-planar and consists of two groups, namely phthalimide and nitroacetophenone groups. The phthalimide group is planar and the bond lengths and angles are within normal ranges [17,18] The C=O bond length [C11-O11, C31-O31 = 1.220(14), 1.201 Å] is typical for arylmethylcetone. The asymmetric unit consists of two crystallographically independent molecules. The dihedral angle between phthalimide and the nitroacetophenone group is 73.84(8)°. The latter is not planar with a maximum deviation of 10.03(3)° in both molecules. Bond lengths and angles are in normal ranges [19]. All hydrogen atoms were positioned geometrically and allowed to ride on their parent atoms with d(Csp2-H) = 0.93 Å or d(Csp3-H)=0.97Å and Uiso(H)=1.2Ueq(C).
Biological assays
Trypanosoma cruzi: The T. cruzi NINOA strain was employed in this study. This strain was isolated from an acute case of Chagas disease in the city of Oaxaca, Mexico. The parasite was maintained in the laboratory in the vector Meccus pallidipennis (Insecta:Hemiptera) and in female white mice.
In vitro trypanocidal assay: Compounds under study were dissolved in dimethyl sulphoxide (DMSO) at a concentration of 10 mg/mL and serial dilutions with distilled water were done to obtain the desired concentrations. All drugs and benznidazole (Bnz) as reference drug were evaluated against bloodstream trypomastigotes obtained by cardiac puncture of infected mice at the peak of parasitemia. Heparin was used as anticoagulant. The infected blood was diluted with sterile 0.85% saline solution and the parasites were adjusted at 1 x 106 bloodstream trypomastigotes/mL. Bioassays were done in 96-well plates. At each well contained was added 195 μL of the trypomastigote suspension and 5 μL of each drug at a final concentration of 5, 10, 50, 100 μg/mL. Control of DMSO was assayed in parallel and the concentration of DMSO never exceed 1%. The plates were incubated at 4°C for 24 h. The trypomastigotes were counted by the method described by Brener [20]. The anti-trypanosome activity was expressed as the percentage of reduction in the number of the treated parasites compared with the control (nontreated parasites). All assays were run in triplicate.
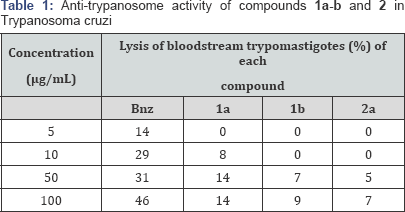
Trypanocidal activity: The compounds considered under this study (1a-b, 2a) where compared with the chemotherapeutic agent of choice in the treatment of chagasic patients benznidazole (Bzn) which showed a lytic activity of 31% on bloodstream trypomastigotes at a concentration of 50 μg/mL and a maximum of 46 % at 100 μg/mL. Thus, the compounds 1a, 1b and 2 showed almost non observable lytic activity (Table 1).
Lectures at 24 h of incubation at 4°C , Inhibition of 2b ref 4.
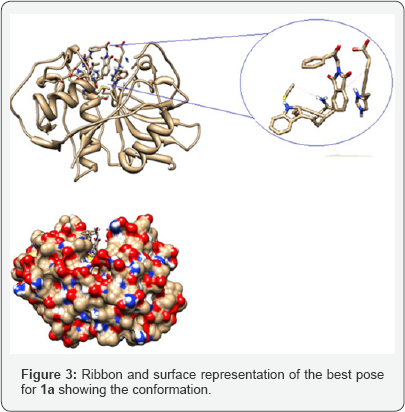
Docking analysis: The conformational analysis of the synthesized ligands 1a-b, 2a-b were carried out in order to find the best binding mode, pose and the interactions between the ligands and the residues. The program used for this study was Autodock program [21] which employs the Lamarckian algorithm and the Chimera program as visualizer system [22]. The enzyme used for the simulation was cruzain (PDB code: 1ME3) and the selected ligands were Gln19, Cys22, Ser24, Cys25, Trp26, Gly66, Asp158, His159 which are key residues present at the active site. The grid boxes have dimensions of 20 x 20 x 20, with spacing of 1.000 and x, y and z value centers of 5.417, 6.89, and 9.036. The first compound analyzed 1a presents three rotatable with binding energy of -4.78 kcal/mol, displaying a conformation with the phtalimide moiety oriented toward the active site as shown in the ribbon and surface representations (Figure 2).
The lowest energy conformer 1a did not show hydrogen bond interactions, however the conformation with binding energy of -2.19 showed an interaction with Cys25 (Figure 3). Ribbon and surface representation of the best pose for 1a showing the conformation and the residues around the ligands. The docking analysis of a-phtalimide-3-nitro-acetophenone 1b presents four rotatable bonds with binding energy -4.29 kcal/mol for the most favored conformation having none hydrogen bond interaction with the selected residues. The ribbon and surface representation shows the ligand 1b assuming a conformation with the phtalamide and benzoyl groups positioned in partial coplanarity without moieties embedded into the active site (Figure 4). Also it was observed a less favored conformation with binding energy of -2.2 showed hydrogen bond interaction with Gly66 (Figure 5).
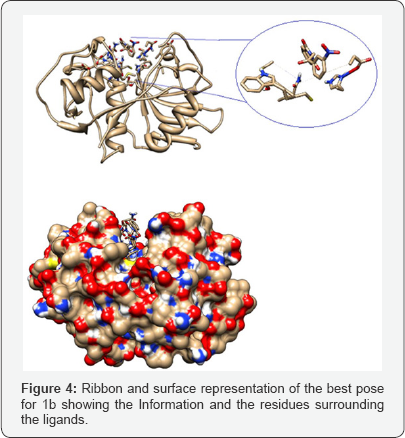
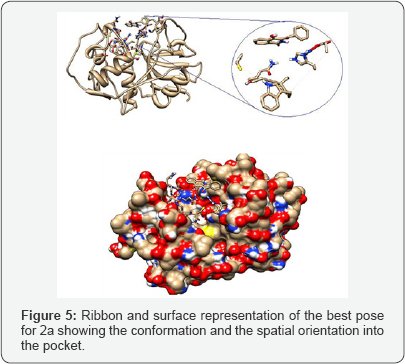
Ribbon and surface representation of the best pose for 1b showing the conformation and the residues surrounding the ligands. The docking analysis of benzoyl isoquinolone 2a presents three rotatable bonds having a binding energy -3.99 kcal/ mol, for the most favored conformation. The minimum energy orientation assumed for 2a shows the isoquinolone moiety positioned almost orthogonal to the benzoyl fragment with none of the fragments embedded into the pocket (Figure 5). Ribbon and surface representation of the best pose for 2a showing the conformation and the spatial orientation into the pocket. The ligand 2b presents four rotatable bonds with binding energy of -3.04 kcal/mol, assuming a none orthogonal conformation with a binding mode opposite with 2a, establishing a hydrogen bridge interaction between the enol group from the ligand and the carbonyl group of the residue Gly66 (Figure 6).

From the docking analysis the scoring functions were determined providing low energy values indicating a likely binding interaction with the residues. However, although the binding energy and ligand efficiency for the phtalimide derivatives 1a-b are from the same order as for the isoquinolone ligands 2a-b and 3 the lysis was not observed. This finding can lead us to the conclusion that the phtalimide moiety is not a suitable pharmacophore for cruzain, although this statement is not conclusive considering the number of intermediates evaluated. In the case of isoquinolones derivatives 2a-b the ligand 2b probed to be substantially more active than the unsubstituted 2a assuming consequently that the presence of the nitro group is critical for activity (Table 2).

Conclusion
We have described the synthesis, trypanocidal inhibition and comparative analysis of phtalimide acetophenones and isoquinolones, observing no trypanocidal inhibition for the phtalimide derivatives (1a-b) and isoquinolone 2a, but a strong inhibition as reported previously for the nitro derivative 2b [4]. We also described the X-ray diffraction of compounds 1b which allowed us to analyze the conformation and hydrogen bonding interaction on the solid state and the docking studies to determine the best pose and interaction with the active site in the target enzyme cruzain.
Conflict of Interest
The authors declare that there is no conflict of interest in the preparation of this article.
Acknowledgement
MBA would like to thank Cofaa-IPN, and SIP-IPN for financial support during the development of this project.
References
- Rivera G, Bocanegra-Garci'a V, Ordaz-Pichardo C, Nogueda-Torres B, Monge A (2009) New therapeutic targets for drug design against Trypanosoma cruzi, advances and perspectives Current Medicinal Chemistry 16(25): 3286-3293.
- Reithinger R, Tarleton RL, Urbina JA, Kitron U, Gurtler RE (2009) Eliminating Chagas disease: challenges and a roadmap. British Medical Journal pp. 338: b1283.
- McGrath ME, Eakin AE, Engel JC, McKerrow JH, Craik CS, et al. (1995) The Crystal Structure of Cruzain: A Therapheutic Target of Chagas' Disease. J Mol Biol 247(2): 251-259.
- Byler KG, Brito-Arias M, Marquez-Navarro A, Nogueda-Torres B, Torres- Bustillos LG, et al. (2012) Identification of benzoylisoquinolines as potential anti-Chagas agents Bioorganic & Medicinal Chemistry 20(8): 2587-2594.
- Romeiro NC, Aguirre G, Hernandez P, Gonzalez M, Cerecetto H, et al. (2009) Synthesis, trypanocidal activity and docking studies of novel quinoxaline-N-acylhydrazones, designed as cruzain inhibitors candidates Bioorganic & Medicinal Chemistry 17: 641-652.
- Zanatta N, Amaral SS, dos Santos JM, de Mello DL, Fernandes LS, et al. (2008) Convergent synthesis and cruzain inhibitory activity of novel 2-(N’-benzylidenehydrazino)-4-trifluoromethyl-pyrimidines. Bioorganic & Medicinal Chemistry 16(24): 10236-10243.
- Trossini GHG, Guido RVC, Oliva G, Ferreira EI, Andricopulo AD (2009) Quantitative structure- activity relationships for a series of inhibitors of cruzain from Trypanosoma cruzi: Molecular modeling, CoMFA and CoMSIA studies. Journal of Molecular Graphics and Modelling 28(1): 3-11.
- Pizzo C, Faral-Tello P, Salinas G, Flo M, Robello C, et al. (2012) Selenosemicarbazones as potent cruzipain inhibitors and their antiparasitic properties against Trypanosoma cruzi Med Chem Commun 3: 362.
- éndez-Lucio O, Romo-Mancillas A, Medina-Franco JL, Castillo R (2012) Computational study on the inhibition mechanism of cruzain by nitrile- containing molecules Journal of Molecular Graphics and Modelling 35: 28-35.
- Choe Y, Brinen LS, Price MS; Engel JC, Lange M, et al. (2005) Development of α-keto-based inhibitors of cruzain, a cysteine protease implicated in Chagas disease. Bioorganic & Medicinal Chemistry 15: 2141-2156.
- Hill JHM (1965) Mechanism of the Gabriel-Colman Rearrangement J Org Chem 30(2): 620-622.
- Brito-Arias M, Castillo-Juarez K, Molins E (2006) 3-benzoyl-4- hydroxyisoquinolin-1(2H)-one Acta Crystallogr, Sect E: Struct Rep Online o5261.
- (1997) Nonius Kappa-CCD Server Software Nonius BV, Delft, The Netherlands,Europe.
- Otwinowski Z, Minor W (1997) Methods in Enzymology, Vol 276, Macromolecular Crystallography, Part A, edited by CW Carter Jr & RM Sweet pp. 307-326.
- Sheldrick GM (2008) A short history of SHELX Acta Cryst 64(1): 112122.
- Farrugia LJ (1997) ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI) J Appl Cryst 30: 565.
- Feeder N, Jones W (1996) Structures of Five-Phthalimidoaliphatic Carboxylic Acids Acta Cryst. C52: 913-919.
- Ng SW (1992) Structure of 1H-isoindole-1, 3 (2H)-dione (phthalimide) Acta Cryst. C48 1694-1695.
- Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG et al. (1987) Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds J Chem Soc Perkin Trans pp. S1-19.
- Brener Z (2010) Revista do Instituto de Medicina Tropical de Sao Paulo, 1962(4): 389-396.
- Sanner MF, Huey R, Dallakyan S, Karnati S, Lindstrom W, et al. (2007) AutoDockTools, version 1.4.5. The Scripps Research Institute, USA.
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. (2004) UCSF chimera-a visualization system for exploratory research and analysis. J Comput Chem 25(13): 1605-1612.






























