Novel Route for Synthesis of Anti - Hyperglycaemic Activity of Thiazolidine 2,4- Dione Derivatives As A Mannich Bases
Ramakrishna Vellalacheruvu*1, R Sai Leela2 and L K Ravindranath1
1Department of Chemistry, SK University, India
2College of Pharmacy, SK University, India
Submission: July 28, 2017; Published: August 21, 2017
*Corresponding author: Ramakrishna Vellalacheruvu; Department of Chemistry, Sri Krishna Devaraya University, Anantapur, Andhra Pradesh, India, Tel: 9493268448; Email: vellalacheruv@gmail.com
How to cite this article: Ramakrishna V, R S Leela, L K Ravindranath. Novel Route for Synthesis of Anti -Hyperglycaemic Activity of Thiazolidine2,4-Dione Derivatives As A Mannich Bases. Organic & Medicinal Chem IJ. 2017; 3(2): 555607. DOI: 007 10.19080/OMCIJ.2017.03.555607.
Abstract
The mannish bases of Thiozolidine 2,4 -dione derivatives has come to lime light due to their multi functional biological activities. Thiazolidine- 2,4- dione is an extensively explored hetero cyclic nucleus for designing of novel agents implicated for a wide variety of pathophysiological conditions, that is, diabetes, diabetic complications ,cancer, arthritis, inflammation, microbial infection, and melanoma. Present work, synthesise quinoline attached imidozoline derivative using (3 +2) cycloaddition via imine of quinoline and TOSMIC. These derivatives were converted to mannich bases of thiozolidine 2,4 one using knoevenagel condensation. The sulfonyl derivatives of thiozolidine 2, 4 -dione were also synthesized and characterized by using alkylation conditions.
Keywords: Imidazole derivatives; Thiozolidine 2,4 one nucleus; TOSMIC; Combi- flash Chromatography
Introduction
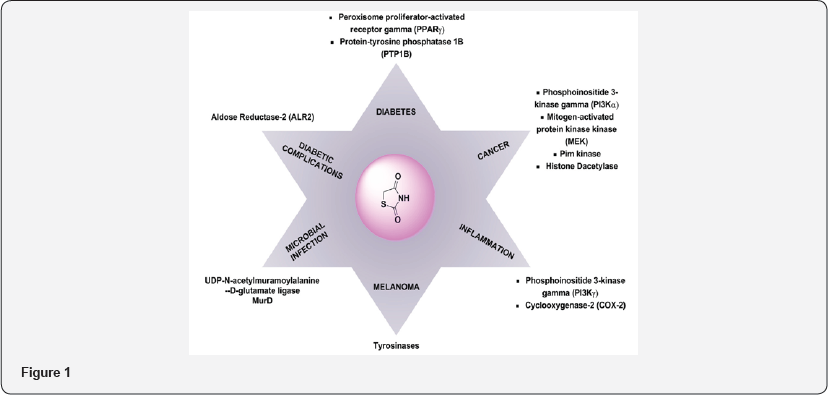
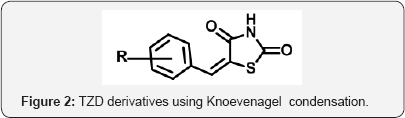
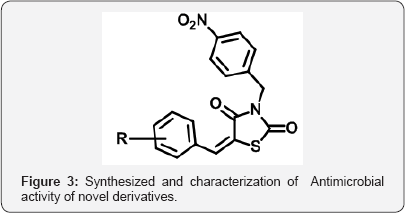
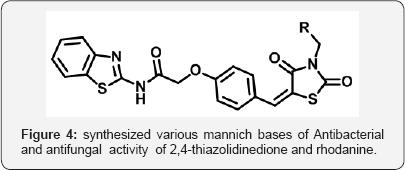
Thiazolidine 2,4 dione (TZD) is a vital nucleolus in heterocyclic chemistry. TZD shows multidirectional phamocodynamical activities such as Anti-hyperglycemic (glitazone drugs), anti-cancer, anti microbial, anti-arthritic. Due to multidirectional pathological actions, huge explored research work has been attempted and still efforts under progress for drug candidates. The metabolic disorder of diabetic is now a day's shows major impact on human beings throughout world. The Thiazolidine 2,4-dione (TZD) derivatives act as a drug candidates such as rosiglitagone, pioglitazone, lobigli tazone, enaglitazone, netoglitazone, ozoline, daragletazone, troglitazone etc. TZD derivatives not only confine for treatment of metabolic disorder diabetic, it o shows as an inflammatory agents, and anti- cancer and for treatment of melanoma. Due to importance of TZD, derivatives many scientists have been developed various routes for synthesis. Om silakari et al. developed different TZD derivatives and evolutes their biological activity (Figure 1). Ivanildo Mangueira, da Silva and co workers [1] developed TZD derivatives using Knoevenagel condensation (Figure 2). Boja Poojary and co workers [2] synthesized and characterization of Antimicrobial activity of novel derivatives (Figure 3). Archana Kapoor and Neha Khare [3] synthesized various mannich bases of Antibacterial and antifungal activity of 2,4-thiazolidinedione and rhodanine (Figure 4). Many routes has been developed for synthesis of 2,4thiozolidinone. The Thiazolidine 2,4 dione having many active sites. Thiazolidine 2,4,dione nucleus numbering is given as fallows (Figure 5).
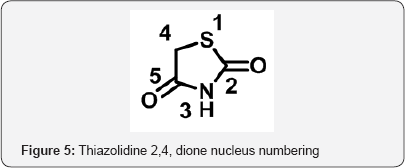
Materials and Methods
All reagents and starting material were procured from commercial sources (Aldrich, Alfa Aesar). Solvents were thoroughly dried before use. THF and toluene were dried using sodium metal and benzophenone.DMF was dried using CaH. The new compounds were fully characterized using Analytical methods like IR, NMR (Bruker). The melting points were recorded using on a (WRS-1A) Digital Melting Point Apparatus without correction. Infrared spectra were taken using an AVATAR 370 FT-IR spectrometer. HNMR, CNMR spectra were recorded with a Bruker spectrometer operating at 400MHz used as a Trimethyl silane reference and values recorded in ppm. The progress of reaction was monitored using TLC system and I2 spray and KMnO4 TLC strain. The crude compounds were purified using column chromatography (100-200 mesh silica) and Combi-flash chromatography. The hydrogenolysis process was carried out using parr shaker [4-19].
Objective of this Research
lated to develop new synthetic route for preparation of the quinoline containing thiozolidin-4- one attached 1,3, 4 oxa diazole nucleus and thiazolidin-4-one attached benz imidazole and benz thiozole and benzoxazole derivatives and thoroughly characterized. The scaffolds of 2-(8-((5-(4- substituted phenyl)-1,3,4-oxadiazol-2-yl)methoxy) quinolin-5-yl)-3-(4-(trifluoromethyl)phenyl)thiazolidin-4- one(7a-h)weresynthesized and characterized.
Experimental Methods
In this research work, we prepared below compounds and mentioned in step wise manner
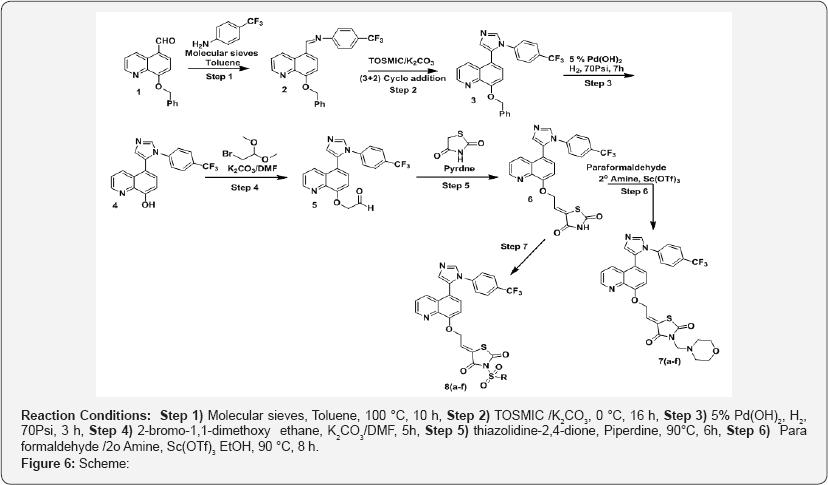
a) Step-1: (Z)-N-((8-(benzyloxy)quinolin-5-yl)methylene)-4- (trifluoromethyl)aniline (2)
b) Step-2: 8-(benzyloxy)-5-(1-(4-(trifluoromethyl) phenyl)- 1H-imidazol-5-yl) quinoline (3).
c) Step-3: 5-(1-(4-(trifluoromethyl) phenyl)-1H-imidazol-5- yl) quinolin-8-ol (4).
d) Step-4: 2-((5-(1-(4-(trifluoromethyl) phenyl)-1H-imidazol- 5-yl) quinolin-8-yl) oxy) acetaldehyde (5).
e) Step-5: 5-(5-(1-(4-(trifluoromethyl)phenyl)-1H-imidazol- 5-yl)quinolin-8-oxy)methylene)thiazolidine-2,4-dione (6).
f) Step-6: 3-(Amine substituted methyl)-5-(2-((5-(1-(4- (trifluoromethyl) phenyl]-1H-imidazol-5-yl]quinolin-8-yl]oxy] ethylidene)thiazolidine-2,4-dione (7a-f).
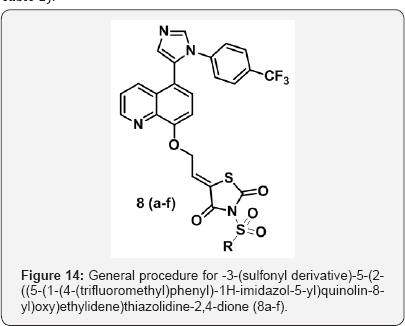
g) Step-7: 3-(sulfonyl-derivatives)-5-(2-((5-(1-(4-(trifluoromethyl) phenyl)-1H-imidazol-5-yl) quinolin-8-yl)oxy)ethylidene)thiazolidine-2,4-dione (8a-f) (Figure 6).
Reaction mechanism for Step 2 (Figure 7)

Step 1: (Z)-N-((8-(benzyloxy) quinolin-5-yl) methylene)-4-(trifluoromethyl) aniline(2)
p class="indent">8-(benzyloxy) quinoline-5-carbaldehyde (10 g, 0.038 mol),4-(trifluoromethyl] aniline (6.5 g, 0.039 mol] in dry toluene ( 100 mL) was added freshly dried molecular sieves and refluxed for 10 h under N2 atm. The progress of reaction was monitored by TLC. After completion of starting material, toluene was evaporated under vacuum to gave crude residue of Compound- 2 (15 g) as a solid ( white colour). The crude was carried to next step (Figure 8).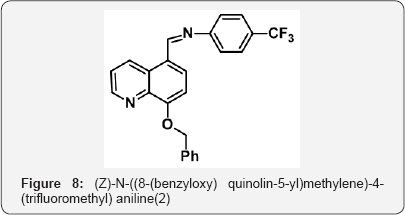
Step 2 : 8-(benzyloxy)-5-(1-(4-(trifluoromethyl) phenyl)-1H-imidazol-5-yl)quinoline(3)
(Z)-N-((8-(benzyloxy) quinolin-5-yl)methylene)-4-(trifluoromethyl) aniline(2) (15 g, 0.036 mol)was dissolved in Dry DMF (80 mL) and cooled to O °C. To that dried K2CO3 (15 g. 108 mol) and Toluene methyl isocyanide (7.02 g, 0.036 mol) was added and warm to room temperature and stirred for 16h. The progress of reaction was monitored by TLC. After completion, reaction mixture was poured in ice cold water (100 mL) and extracted with EtOAc ( 3 x 100 mL). The organic layer was separated and washed with brine solution, dried over anhydrous Na2SO4, filtered and evaporated under vacuum to give crude residue. The obtained crude product was purified by column chromatography (100-200 mesh silica, Eluent: 80% EtOAc-Pet Ether) isolated 8-(benzyloxy)-5-(1-(4-(trifluoromethyl) phenyl)- 1H-imidazol-5-yl)quinoline(3) (10 g, yield: 64%) as a solid (Pale yellow colour). M.p. 252-255 °C. IR (KBr, cm-1): 3030, 1440, 1520, 1005, 691, 655. HNMR (d6-DMSO, 400 mHz): 5.2 (s, 1H, -CH2), 7.1 (m, 3H), 7.3-7.5 (m, 7H), 7.6-7.7 (m, 4H), 7.85(d, 1H), 8.36(d, 1H), 8.85(d, 1H ) (Figure 9).

Step 3 : 5-(1-(4-(trifluoromethyl)phenyl)-1H-imidazol- 5-yl)quinolin-8-ol (4)
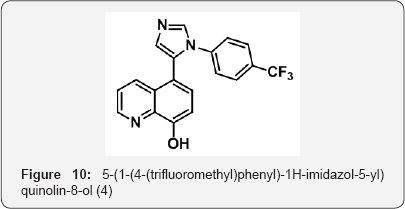
8-(benzyloxy)-5-(1-(4-(trifluoromethyl)phenyl)-1H- imidazol-5-yl)quinoline(3) (10 g, 0.022 mol ) in MeOH (100 mL) was added 5% Palladium hydroxide on carbon (1 g, cat) and hydrozinated at 70 Psi under parr shaker for 3 h at room temperature. The progress of reaction was monitored by TLC. After completion, reaction mixture was filtered on cellite bed and thoroughly washed with MeOH (2 x 75 mL). The MeOH layer were collected and evaperated under vaccum to gave 5-(1-(4-(trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8- ol (4)(7g, yield : 86%) as a solid (white colour)). M.p. 280-285 °C. IR (KBr, cm-1): 3620, 3014, , 1525, 1050, 691, 620 .1HNMR ( d6-DMSO, 400 mHz) : 6.5 (brs, 1H),7.1 (m, 2H), 7.3 (d, 2H), 7.63 (m, 4H),7.8(d,1H), 8.35(d, 1H), 8.8(d, 1H) (Figure 10).
Step 4 : 2-((5-(1-(4-(trifluoromethyl)phenyl)-1H- imidazol-5-yl)quinolin-8-l)oxy)acetaldehyde (5)
5-(1-(4-(trifluoromethyl) phenyl)-1H-imidazol-5-yl) quinolin-8-ol (4) (7 g, 0.017 mol) in Dry DMF (70 mL) was added K2CO3 (9.7 g, 0.07 mol, 4 eq) and stirred at rt for 30 min. To that a solution of 2-bromo-1,1-dimethoxy ethane( 1.2 eq) in DMF (20 mL) was added drop wise at 0°C and stirred for 5h. The progress of reaction was monitored by TLC. After completion, reaction mixture was filtered on cellite bed and washed with DMF (10 mL). The Reaction mixture was poured in ice cold water (200 mL) and stirred for 20 min. The reaction mixture was acidified aq NaHSO3 solution up to PH-5 and extracted with EtOAc (2 x 200 mL). The aqueous layer was collected and basified up to PH-8 with sat aq NaHCO3 sol. The aqueous layer was extracted with EtOAc (3 x 100 mL). The organic layer were collected and dried over anhydrous Na2SO4, filtered and evaporated under vacuum to gave 2-((5-(1-(4-(trifluoromethyl)phenyl)-1H-imidazol-5- yl)quinolin-8-yl)oxy) acetaldehyde (5) (5g) as a solid( white colour). M.p. 200-205 °C. IR (KBr, cm-1): 3602, 3014, 1712, 1646, 1503, 1050, 691, 644.1HNMR (d6-DMSO, 400 mHz) : 5.2 (s, 2H), 7.1 (m, 2H), 7.3(d, 2H),7.9(d, 1H), 8.36(d, 1H), 8.82(d, 1H), 9.6(s, 1H) (Figure 11).
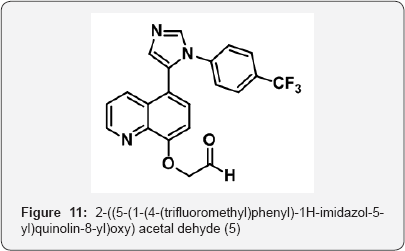
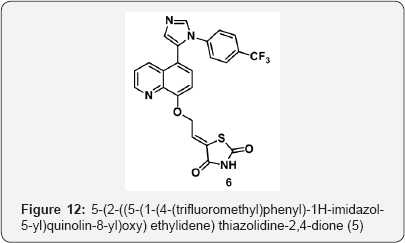
Step 5: 5-(2-((5-(1-(4-(trifluoromethyl)phenyl)- 1H-imidazol-5-yl)quinolin-8-yl)oxy) ethylidene) thiazolidine-2,4-dione (5)
To a mixture of 2-((5-(1-(4-(trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8-yl)oxy) acetaldehyde (5) (5g, 0.012 mol), thiazolidine-2,4-dione (1.62 g, 0.013 mol) in EtOH (50 mL) was added piperdine ( 2 mL) and heated at 90 °C for 6 h. The progress of reaction was monitored by TLC. After completion, Reaction mixture was evaporated under vacuum to gave crude residue. The residue was diisolve in water(100 mL) and filtered under vaccum and dried to gave (Z]-5-(2-((5-(1-(4- (trifluoromethyl) phenyl)-1H-imidazol-5-yl)quinolin-8-yl)oxy) ethylidene) thiazolidine-2,4-dione (6) ( 5.5 g, Yield: 88% ) as a solid ( brown colour).M.p:240-243 °C. IR (KBr, cm-1): 3050, 1725, 1650, 1503, 1050, 691, 644.1HNMR ( d6-DMSO, 400 mHz) : 4.6 (dd, 1H), 4.61(dd, 1H), 6.15 (dd, 1H), 7.12 (m, 2H), 7.3 (d, 2H), 7.6-7.65 (m, 4H), 7.9(d, 1H), 8.4(d, 1H), 8.6 (brs, 1H), 8.87 (d, 1H) (Figure 12).
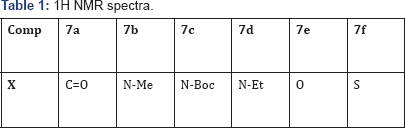
Step 6 : 3-( Amino substituted methyl)-5-(2-((5-(1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)qumolm- 8-yl)oxy)ethylidene)thiazolidine-2,4-dione &7(a-f)
To a mixture of (Z)-5-(2-((5-(1-(4-(trifluoromethyl) phenyl]- 1H-imidazol-5-yl)quinolin-8-yl)oxy)ethylidene) thiazolidine-2,4- dione (6) ( 500 mg, ), 2o Amine (1.1 eq), Para formal dehyde (3 eq) in EtOH (50 mL) was added Sc(OTf)3 (0.1 eq) and heated for 8 h. The progress of reaction was monitored by TLC. After completion, EtOH was evaporated under vaccum to give crude product. The crude was purified by reverse-phase column chromatography (C18 silica, Eluent: 30% ACN-MeOH-H2O,0.01% TFA) isolated (Z)-3-(Amino substituted methyl)-5-(2-((5- (1-(4-(trifluoromethyl]phenyl]-1H-imidazol-5-yl)quinolin-8-yl) oxy)ethylidene) thiazolidine-2,4-dione &7(a-f). 1H NMR spectra of 7(a-f) was given below Table 1 & Figure 13.
a) 3-((4-oxopiperidin-1-yl)methyl)-5-(2-((5-(1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8-yl) oxy)ethylidene)thiazolidine-2,4-dione (7a): M.p. 280-283 °C. IR (KBr, cm-1): 3050, 3010, 1720, 1655, 1600, 1320,770,620,. 1HNMR ( d6-DMSO, 400 mHz) : 2.4 (t, 4H), 2.8 (t, 4H), 4.65 (s, 2H,), 4. 68(dd,2H), 4.7 (dd,1H), 5.1(d, 2H), 6.8 (dd, 1H), 7.1 (m, 1H), 7.3 (d, 2H), 7.6 (m, 4H), 8.0 (d, 1H), 8.43 (d, 1H), 8.81(d, 1H). 13CNMR ( d6-DMSO, 400 mHz) : 45, 53, 65, 108, 120, 122, 124, 124.5, 131, 135, 139, 145,150,164, 173, 190.
b) 3-((4-methylpiperazin-1-yl)methyl)-5-(2-((5-(1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8-yl) oxy)ethylidene)thiazolidine-2,4-dione (7b): M.p. 290-292 °C. IR (KBr, cm-1): 3350, 3050, 1660,1610, 1320,750, 6251HNMR ( d6-DMSO, 400 mHz) : 2.3 (s, 3H), 2.4 (d, 4H), 2.45 (d, 4H), 4.63 (s, 2H,), 4.66 (dd, 1H),4.67 (dd,1H), 6.81 (dd, 1H), 7.1 (m, 2H), 7.32 (d, 2H), 7.6 (m, 4H), 8.03 (d, 1H), 8.44 (d, 1H), 8.81(d, 1H). C-NMR ( d6-DMSO, 400 mHz) : 47, 53, 58, 65, 107, 121, 122, 124, 124.5, 127, 130, 134, 138, 145,150,163, 174.
c) tert-butyl4-((2,4-dioxo-5-(2-((5-(1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8-yl) oxy)ethylidene)thiazolidin-3-yl)methyl)piperazine-1- carboxylate (7c): M.p. 260-2262 °C. IR (KBr, cm-1): , 3014, 1713, 1650, 1620, 1505, 1310, 1050, 698, 655, HNMR ( d6-DMSO, 400 mHz) : 1.4 (s, 9H ), 2.5 (t, 4H), 3.1(t, 4H), 2.45 (d, 4H), 4.5 (s, 2H,),4.68 (dd, 1H),4.69 (dd,1H), 6.83 (dd, 1H), 7.1 (m, 2H), 7.32 (d, 2H), 7.62 (m, 4H),7.9 (d, 1H), 8.42 (d, 1H), 8.82(d, 1H). 13C-NMR ( d6-DMSO, 400 mHz) : 31, 44, 52, 65, 78, 107, 121, 124, 127, 130, 132, 139, 145,154,162, 174.
d) 3-((4-ethylpiperazin-1-yl)methyl)-5-(2-((5-(1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8-yl) oxy)ethylidene)thiazolidine-2,4-dione (7d): M.p. 290-292 °C. IR (KBr, cm-1): 3350, 3020, 1680,1620, 1330,750, 6251HNMR ( d6-DMSO, 400 mHz) : 1.2 (t, 3H ), 2.5 (m, 10H), 4.5 (s, 2H,), 4.67(d, 1H), 4.68(d, 1H), 6.80 (dd, 1H), 7.1 (m, 2H), 7.3(d, 2H), 7.62 (m, 4H),7.8 (d, 1H), 8.42 (d, 1H), 8.82(d, 1H). C-NMR ( d6- DMSO, 400 mHz) : 14, 50, 53,58,65, 108, 122, 124, 125, 130, 132, 145,149,165, 174.
e) 3-(morpholinomethyl)-5-(2-((5-(1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8-yl) oxy)ethylidene)thiazolidine-2,4-dione (7e): M.p. 280-282 °C. IR (KBr, cm-1): 3016, 1720, 1650, 1503, 1300, 1050, 695, 650.1HNMR ( d6-DMSO, 400 mHz) : 2.6 (t, 4H ), 3.7 (t, 4H), 4.5 (s, 2H,), 4.68 (dd, 1H), 4.69 (dd,1H), 6.80 (dd, 1H), 7.15 (m, 2H), 7.25(d, 2H), 7.62 (m, 4H),7.9(d, 1H), 8.38 (d, 1H), 8.89(d, 1H). C-NMR (d6-DMSO, 400 mHz) : 53,65, 66,107, 123, 124, 125, 130, 132, 139, 145,149,164, 178.
f) 3 - (thi o mo rpho lino methyl) - 5 -(2-((5-(1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8-yl) oxy)ethylidene)thiazolidine-2,4-dione (7f): M.p. 272-275 °C. IR (KBr, cm-1): 3080, 1730, 1645, 1520, 1310, 1125, 670, 660, 1HNMR ( d6-DMSO, 400 mHz) : 2.6 (t, 4H ), 2.8(t, 4H), 4.51 (s, 2H,), 4.66 (dd, 1H),4.67 (dd, 1H), 6.81 (dd, 1H), 7.15 (m, 2H), 7.25(d, 2H), 7.62 (m, 4H),7.9(d, 1H), 8.37 (d, 1H), 8.86(d, 1H). C-NMR ( d6-DMSO, 400 mHz) : 27,58,63, 106, 122,123, 124, 125, 130, 131, 135,139,145,149,164, 174.
Step 7: General procedure for -3-(sulfonyl derivative)- 5-(2-((5-(1-(4-trifluoromethyl)phenyl)-1H-imidazol- 5-yl)quinolin-8-yl)oxy)ethylidene)thiazolidine-2,4- dione (8a-f)
5-(2-((5-(1-(4-(trifluoromethyl)phenyl)-1H-imidazol-5-yl) quinolin-8-yl)oxy)ethylidene) thiazolidine-2,4-dione (500 mg,1.8mmol) in Dry DMF(5 mL) was added NaH (3 eq) at 0 °C under N2 atm and stirred for 1h. To that sulfonyl chloride (1.1 eq) was added and stirred for 5h.The pogress of reaction was monitored by TLC. The reaction mixture was poured in aq sat NaHCO3 and stirred for 15 min. The aq layer was extracted with 10% MeOH-CHCl3 (3x 25 ml) and dried over anhydrous Na2SO4, filtered and evaporated under vaccum to gave crude product. The crude product was purified by Column chromatography (100-200 mesh silica) isolated 3-(sulfonyl derivative)-5-(2-((5- (1-(4-(trifluoromethyl) phenyl)-1H-imidazol-5-yl)quinolin-8-yl) oxy) ethylidene) thiazolidine-2,4-dione (8a-f) (Figure 14 and Table 2).
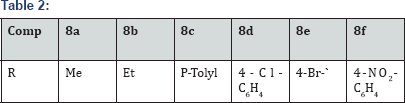
i. 3-(methylsulfonyl)-5-(2-((5-(1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8-yl) oxy)ethylidene)thiazolidine-2,4-dione(8a): M.p.280-283°C. IR (KBr, cm-1): 3040, 3025, 1730, 1645,1600, 1320,1070,770,715620,.1HNMR ( d6-DMSO, 400 mHz) : 2.8 (s, 3H), 4.67 (dd, 1H),4.68 (dd,1H), 6.8 (dd, 1H), 7.1 (m, 2H), 7.3(d, 2H), 7.6 (m, 4H),7.8 (d, 1H), 8.4 (d, 1H), 8.81(d, 1H). CNMR ( d6-DMSO, 400 mHz) : 42, 64, 106, 121, 122, 124, 124.5, 126,130, 135, 138,139, 145, 148, 164, 173.
ii. 3-(ethylsulfonyl)-5-(2-((5-(1-(4-(trifluoromethyl) phenyl)-1H-imidazol-5-yl)quinolin-8-yl)oxy)ethylidene) thiazolidine-2,4-dione(8b): M.p.:88-290 °C. IR (KBr, cm-1): 3040, 3025, 1730, 1645,1603, 1325, 1075,772,715 620,.1HNMR ( d6-DMSO, 400 mHz) : 1.3 (t, 3H), 3.45 (q, 2H), 4.65 (dd, 1H),4.67 (dd,1H), 6.8 (dd, 1H), 7.13 (m, 2H), 7.32(d, 2H), 7.65 (m, 4H), 7.8 (d, 1H), 8.41 (d, 1H), 8.82(d, 1H). 13CNMR ( d6-DMSO, 400 mHz) : 10, 52, 63, 106, 121, 122, 124, 124.5, 126,130, 135, 138,139, 145, 148, 164, 173.
iii. 3-tosyl-5-(2-((5-(1-(4-(trifluoromethyl) phenyl)-1H-imidazol-5-yl)quinolin-8-yl)oxy) ethylidene) thiazolidine-2,4-dione(8c): M.p. 300-302 °C. IR (KBr, cm-1):3050, 3030, 1735, 1640,1603, 1325, 1075,770,715 620.1HNMR ( d6-DMSO, 400 mHz) : 2.3 (s, 3H), 4.68 (dd, 1H), 4.69 (dd, 1H), 6.8 (dd, 1H), 7.13 (m, 2H), 7.32(d, 2H),7.4 (d, 2H), 7.65 (m, 4H),7.7 (d, 2H), 7.8 (d, 1H), 8.42 (d, 1H), 8.85(d, 1H). 13CNMR ( d6-DMSO, 400 mHz) : 20, 64, 107, 121, 122, 123, 124, 124.5,126,128, 130,132, 133 135, 138,139, 145, 148, 155, 165, 175.
iv. 3-((4-chlorophenyl)sulfonyl)-5-(2-((5-(1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8- yl)oxy)ethylidene)thiazolidine-2,4-dione (8d): M.p. 290292 °C. IR (KBr, cm-1): 3070, 3020, 1720, 1620,1530,1325, 1080,770,720, 655.1HNMR ( d6-DMSO, 400 mHz) : 4.68(dd, H), 4.69 (dd, 1H), 6.85 (dd, 1H), 7.15 (m, 2H), 7.28(d, 2H),7.4(d, 2H), 7.63(m, 6H),7.8(d, 2H), 7.95(d, 1H), 8.5 (d, 1H), 8.87(d, 1H). 13CNMR ( d6-DMSO, 400 mHz) : 64, 108, 121, 122, 123, 124,
124.5, 126,128, 130,132, 133 135, 139, 145, 149, 155, 162, 173.
v. 3- ((4-bromophenyl)sulfonyl)-5 - (2- ((5 - (1-(4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8- yl)oxy)ethylidene)thiazolidine-2,4-dione (8e): M.p. 298300 °C. IR (KBr, cm-1): 3080, 3025, 1720, 1623,1530,1325, 1085,770,725, 655.1HNMR ( d6-DMSO, 400 mHz) : 4.68(dd, 1H), 4.69 (dd,1H), 6.87 (dd, 1H), 7.18 (m, 2H), 7.28(d, 2H),7.6(m, 4H), 7.88(m, 5H), 8.4 (d, 1H), 8.8(d, 1H). 13CNMR (d6-DMSO, 400 mHz): 64.2, 107, 121, 122, 123, 124, 124.5, 126,128, 130,132, 134, 136, 139, 146, 149, 155, 163, 174.
vi. 3 - ((4-nitrophenyl)sulfonyl) - 5 - (2- ((5- (1- (4- (trifluoromethyl)phenyl)-1H-imidazol-5-yl)quinolin-8-yl) oxy)ethylidene)thiazolidine-2,4-dione(8f): M.p. 305-308 °C. IR (KBr, cm-1): 3070, 3028, 1720, 1623,1535, 1400, 1328, 1085,770,730, 655. 1HNMR ( d6-DMSO, 400 mHz) : 4.68(dd, 1H),4.6 9(dd, 1H), 6.87 (dd, 1H), 7.18 (m, 2H), 7.28(d, 2H),7.6(m, 4H), 7.8(d, 1H), 8.12 (d, 2H), 8.4 (m, 3H), 8.85(d, 1H). 13CNMR ( d6-DMSO, 400 mHz) : 64.2, 107, 121, 122, 123, 124, 124.5,126,128, 130,132, 134, 136, 139, 142, 146, 149, 151155, 164,174.5.
Conclusion
In this research work we successfully synthesized and characterization, mannich bases of quinoline attached imidazoline thiozolidine 2,4-one derivatives . We are planning to these derivatives check for biological evolution. The biological evolution details will include next journal.
Acknowledgement
I sincerely thankful to my guide and co-workers, and Department of Chemistry, Sri Krishna devaraya University, (Anantapur,) and Gvk Bio Sciences for providing laboratory and analytical facilities.
References
- Ioana Mirela Vasincu, Maria Apotrosoaei , Andreea-Teodora Panzariu (2014) Synthesis and Biological Evaluation of New 1,3-Thiazolidine-4- one Derivatives of 2-(4-Isobutylphenyl)propionic Acid. Molecules 19: 15005-15025.
- Navriti Chadha, Malkeet Singh Bahia, Maninder Kaur, Om Silakari (2015) Bioorganic & Medicinal Chemistry 23: 2953-2974.
- Ivanildo Mangueira da Silva, Joao da Silva Filho, Priscila Brandao Gomes da Silva (2014) Synthesis and Antimicrobial Activities of 5-Arylidene- thiazolidine-2,4-dione Derivatives. Med Research international 2014: 1-8.
- H Sharghi , R Khalifeha, F Moeinia, MH Beyzavia, A Salimi Benic, MM Doroodmand (2011) Mannich Reaction of Secondary Amines, Aldehydes and Alkynes in Water Using Cu/C Nanoparticles as a Heterogeneous Catalyst. J Iran Chem Soc 8: 89-103.
- Archana Kapoor, and Neha Khare (2016) Anti-hyperglycemic evaluation of 2,4-thiazolidinedi one and rhodanine derivatives. Der Pharmacia Lettre 8(12):143-148.
- Andreea-Teodora Panzariu, Maria Aprotosoaei, Lenuta profire (2016) Chem Cent J. 10.
- Faiyazalam M Shaikh, Navin B Patel, Dhanji Rajani (2013) Indian jounal of pharmacy and Biotecnology 1: 496.
- Devprakash, Udaykumar A Bhoi (2011) A complete review of thiazolidine-4-ones. Journal of Pharmacy Research 4 (7): 2436-2440.
- (1990) European Journal of Medicinal chemistry 25(7): 569-579.
- BA Bhaviskar, SS Khadabadi, SL Deore (2013) Journal of Chemistry 2013 (2013): 1-6.
- Tribhuvan Singh, Deepak Khobragade (2014) Synthesis and Evaluation of Thiazolidine-4-One for their Antibacterial Activity. Journal of Pharmaceutical Science and Bioscientific Research 4(1): 110-113.
- Kishan D Patel, Chhaganbhai N Patel, and Grishma M Patel (2016) Microwave Assisted Synthesis and Antidiabetic Activity of Novel 5-[4-(Substituted) Benzylidine]Thiazolidine-2,4-Dione. Med Chem (Los Angeles) 6-10.
- Mithun Rudrapal, Dipak Chetia (2016) Asian Journal of Organic and Medicinal Chemistry, 1(2): 53-54.
- Arthur Harutyunyan (2017) Organic and Medicinal Chemistry IJ 3(1): 1-2.
- Prajwal L Lobo, Boja Poojary, Manjunatha K, Prathibha A, N Suchetha Kumari, et al. (2012) Novel thiazolidine-2,4-dione mannich bases:Synthesis, characterization and antimicrobial activity. Der Pharma Chemica 4 (3): 867-871.
- Haitao Ji, Qing Jing, Jinwen Huang, Richard B Silverman (2012) Tetrahedron 68(5): 1359-1366.
- Nicolas Primas, Pierre Verhaeghe, Anita Cohen, Charline Kieffer , Aurelien Dumetre, et al. (2012) A New Synthetic Route to Original Sulfonamide Derivatives in 2-Trichloromethylquinazoline Series: A Structure-Activity Relationship Study of Antiplasmodial Activity. Molecules 17: 8105-8117.
- Faiyazalam, M Shaikh, Navin B Patel, Dhanji Rajani (2013) Indian Journal of Research in Pharmacy and Biotechnology 1(4): 496.
- Archana Kapoor, Neha Khare (2016) Anti-hyperglycemic evaluation of 2,4- thiazolidinedione and rhodanine derivatives. Der Pharmacia Lettre 8(12): 143-148.






























