Promoted by Samarium Reaction of (3r,4r)-3-((lr)-l-{[Tert-Butyl(dimethyl)Silyl]oxy}Ethyl)-4-Acetoxy-Azetidin-2-one with Methyl 2-Bromopropianate. Unusual Decyclization of Azetidin-2-one Derivative in Approaches to Carbapenems Analogues
Zuleykha R Valiullina1, Lidiya S Khasanova1, Natalia K Selezneva1, Yu N Belokon2, Leonid V Spirikhin1 and Mansur S Miftakhov1*
1Ufa Institute of Chemistry of the Russian Academy of Sciences, Russia
2AN Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, Russia
Submission: June 20, 2017; Published: June 26, 2017
*Corresponding author: Mansur S Miftakhov, Ufa Institute of Chemistry of the Russian Academy of Sciences, Russia, 450054, Ufa, Prosp. Octyabrya 71; Email: bioreg@anrb.ru
How to cite this article: Zuleykha R V, Lidiya S K, Natalia K S, Y N Belokon, Leonid V S. Promoted by Samarium Reaction of (3r,4r)-3-((1r)-1-{[Tert-Butyl(dimethyl)Silyl]oxy}Ethyl)-4-Acetoxy-Azetidin-2-one with Methyl 2-Bromopropianate. Unusual Decyclization of Azetidin-2-one Derivative in Approaches to Carbapenems Analogues.Organic & Medicinal Chem IJ. 2017; 2(5): 555597. 10.19080/omcij.2016.02.555597
Abstract
The reaction of the title compound 1 with Sm-reagent prepared from powdered Sm, catalytic amounts of I2 and methyl 2-bromopropionate in THF, leads to the anomalous substituted product 3. The alkylation of the last compound with methyl bromoacetate gives methyl 2-[(2S,3S)- 3-((1R)-1-{[tert-butyl(dimethyl) silyl]oxy}ethyl)-1-(2-methoxy-2-oxoethyl)-4-oxoazetidine-2-yl]-2-methyl-3-oxopentanoate 5 which under the action of NaHMDS in THF at -78° undergoes fragmentation with a disconnection of N1-C4-bond and the formation of acyclic amide 7. Possible stepwise formation routes of 3 and 7 are discussed.
Introduction
Antibiotics of β-lactam series are one of the most popular drugs against infectious diseases. However, microorganisms quickly produce resistance against the used drugs. Now this problem is not solved, but the time of production of this resistance can be increased by introducing new compounds into practice or by modifying the known ones [1,2]. The key block 2 used in the synthesis of practically important 1β-methylcarbapenems (meropenem, ertapenem, doripenem and etc) has been obtained by alkylating azetidinone 1 with Zn-, Li-, B-, Sn- enolates of propionic acid derivatives (amides, thioethers, thia- and oxazolidones, etc.) [3].
We did not find any literature data on the reaction of 1 with Sm-enolates of propionic acid esters. In this paper, in order to obtain new structures, we studied the Sm-promoted Reformat sky Reaction 1 and methyl 2-bromopropianate was carried reaction of azetidin-2-one 1 [4] with methyl 2-bromopropionate.
Results and Discussion
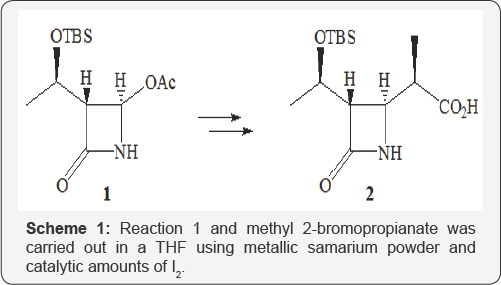
Reaction 1 and methyl 2-bromopropianate was carried reaction of azetidin-2-one 1 [4] with methyl 2-bromopropionate. out in a THF using metallic samarium powder and catalytic amounts of 12 [5]. After the initial azetidinone 1 was consumed, the reaction mass was quenched with aq. NH4Cl. The major alkylation product 3 was isolated in a 70% yield as a 2:1 mixture of diastereoisomers differing by the configurations of the side chain center. The side product azetidinone 4 [6] was isolated in a 5% yield. The subsequent use of 3 was planned according to the traditional methodology for the synthesis of carbapenems from 1 [7-9] through N-alkylation steps of 3 with methyl bromoacetate followed by intramolecular Dieckmann cyclization of adduct 5 to produce precursor 6 (Scheme 1).
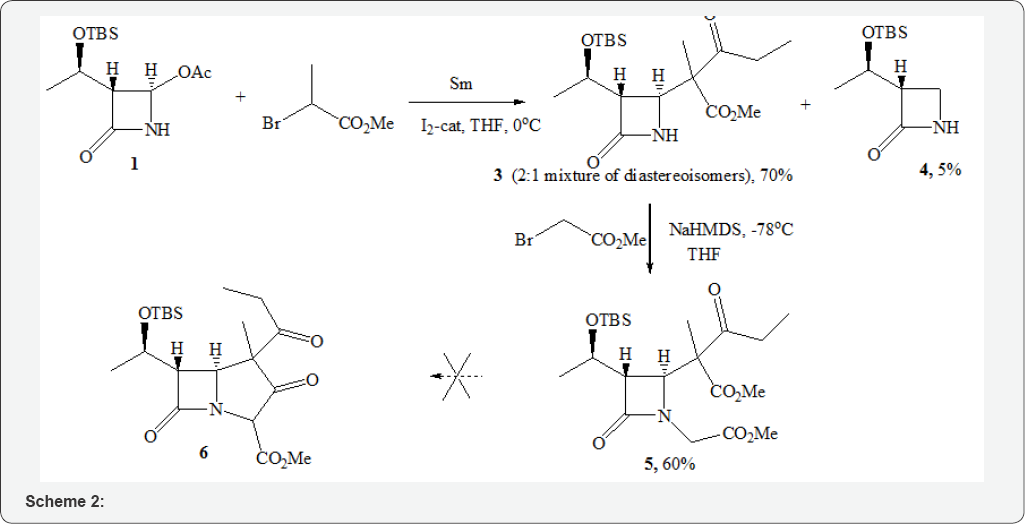
As expected, the alkylation step of 3 with methyl bromoacetate proceeded smoothly with a good yield, leading to 5 as the inseparable (SiO2) mixture of diastereomers in a ratio of 2: 1. An attempted intramolecular cyclization of 5 (NaHMDS,THF,-78°C) failed to produce 6. Instead, a rapid formation of a 1:1 diastereomeric mixture of acyclic amides 7 was observed (Scheme 2).
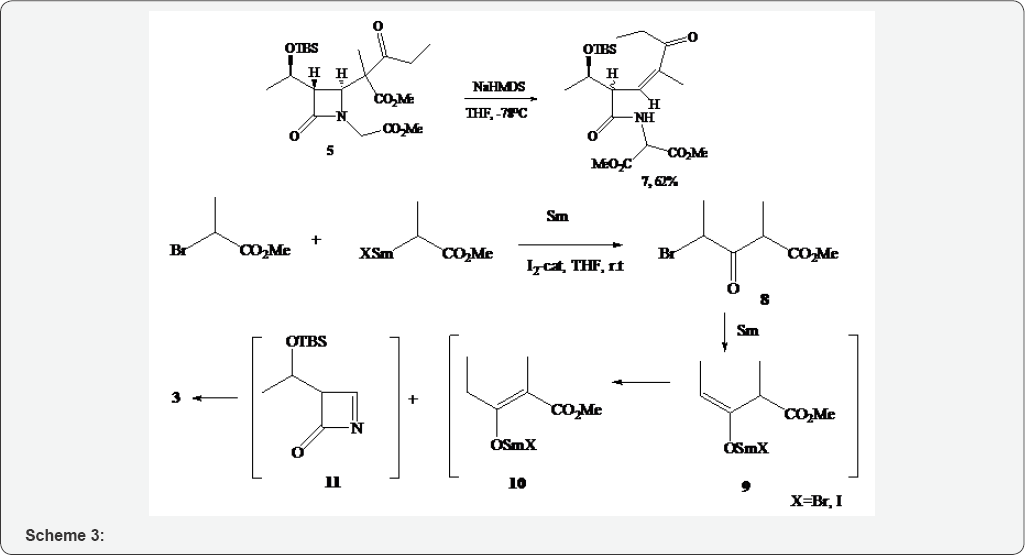
Thus, there was an unusual decyclization of azetidinone 5 at the N1-C4 bond occurring in the reaction. To the best of our knowledge, there were no precedents described for 5 decyclizations in literature. The possible mechanisms of 3 and 7 formation are also of synthetic interest. Obviously, the generation of the Sm reagent from methyl 2-bromopropianate in the synthesis of 3 was preceded by the Claisen-type condensation of two molecules of the bromoester with the formation of β-ketoester 8 (Scheme 3). The generation of enolate 9 with the removal of Br led to stable enolate 10. The latter smoothly reacted with inline 11 formed under the experimental conditions from 1 to give 3.
Numerous examples of the SmI2-promoted Reformatsky type of inter- and intramolecular reactions of α-halo ketones and α-halo-ethers [10-14] have been described in the literature. Thus, the reactions of ethyl α-bromoacetate and α-bromopropionate with Sml2 proceed with the generation of expected Sm reagents that are trapped with carbonyl compounds or isolated as selfcondensation products (β-keto esters)[15-18]. In our case, the nature of the Sm-reagent is slightly different, because instead of Sml2 we used metallic samarium and catalytic amounts of l2 according to the method of the Banik and Basu [5]. All this influence on the results of the reaction, for example, the formation of 4 the product of the reduction of the intermediate imine 11.
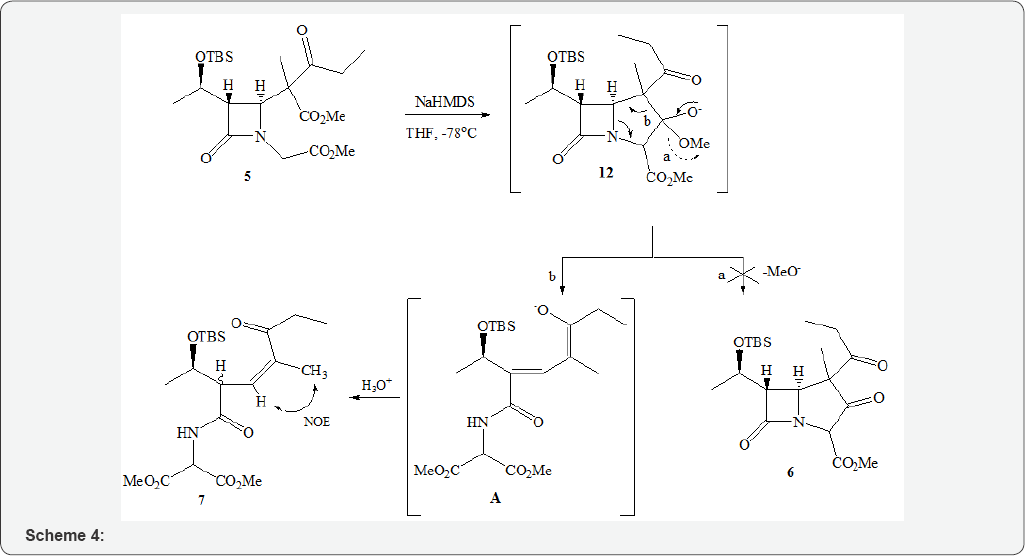
A possible mechanism of 7 generation is presented in Scheme 4. The transient carbanion 12 generated from 5 with NaHMDS can be fragmented in directions a or b. The classical variant (a) with the release of the methoxide anion and the formation of the ketone 6 was not realized and 12 undergoes disintegration by path b, leading after aqueous treatment of the reaction mixture to the acyclic amide 7 (Scheme 4). The driving force for fragmentation 12 by path b is the removal of steric hindrance and the formation of a thermodynamically advantageous enone system 7. Protonation in the acyclic structure A is not stereoselectively and from the initial 2:1 mixture of diastereomers 5, a 1:1 mixture of diasteroisomers of amide 7 was obtained. Any possible isomers at the C=C bond were not formed, and the NOESY spectrum of 7 established its E-configuration evidenced by CH3 and the proton at the double bond interaction.
Conclusion
In conclusion, the Sm-promoted reaction of 1 with methyl 2-bromopropionate led to formation of the substitution product 3 which was different from what could be expected had Sml2 been applied. We associate this course of the reaction with the nature of the Sm reagent, i.e. by generating the rearranged enolate 12. The described unusual variant of fragmentation of 5 under the action of NaHMDS, leading to acyclic lactams 7, is of synthetic interest.
Experimental Section
General
The IR spectra were recorded on a Shimadzu IR Prestige-21 spectrometer from samples prepared as films or mulls in mineral oil. The *H and 13C NMR spectra were recorded on a Bruker AM- 300 (300.13 (*H) and 75.47 (13C) MHz) and Bruker Avance-500 instruments (500.13 1H) and 125.77 (13C) MHz) relative to the residual proton or carbon signals of the deuterated solvent (CHCl3, δ 7.27 ppm; CDCl3, δC 77.00 ppm). The mass spectra (positive electrospray ionization) were obtained on a Shimadzu LCMS-2010EV instrument (samples were injected as solutions in CH3CN with a syringe; eluent acetonitrile-water, 95:5) The progress of reactions was monitored by TLC on Sorbfil plates; spots were detected by treatment with a 10% solution of 4-methoxybenzaldehyde in ethanol containing sulfuric acid.
Sm-promoted Reformat sky reaction of azetidin-2-one 1 with methyl 2-bromopropionate
Methyl 2-bromopropionate (0.35 g, 2.10 mmol) was added drop wise to samarium (0.30 g, 2.10 mmol) (preactivated by heating with 18 mg (0.07 mmol) of iodine) in 7.0 mL dry THF under an argon atmosphere at room temperature. A dark blue color was generated within 0.5-1 h. The azetidin-2-one 1 (0.20 g, 0.70 mmol) was added to the mixture at 0°C. The reaction was stirred for 30 min at the same temperature and then was quenched with saturated solution of NH4Cl. THF was evaporated and the resulting mixture was extracted with ethyl acetate, dried over magnesium sulfate and evaporated to dryness. The residue was purified by column chromatography (silica gel, petroleum ether - ethyl acetate, 8:2 → 7:3) to afford the ether 3 (0.18 g, 70%) and azetidinone 4 (8 mg, 5%).
I. Methyl 2-[(2S,3S)-3-((1R)-1-{[tert-butyl(dimethyl) silyl]oxy}ethyl)-4-oxoazetidin-2-yl]-2(R,S)-methyl-3- oxopentanoate 3: Rf 0.20 (petroleum ether - ethyl acetate, 7:3). White crystal, mp. 84-86 °C. IR, υ, cm-1: 3181, 2921, 1764, 1747, 1716, 1462, 1374, 1252, 1076, 837, 776. Mixture of C2- isomers in a 2:1 ratio (NMR 1H on the intensity of singlet C2-CH3 signals). 1H NMR (500 MHz, CDCl3): δ= 0.05 (s, 6H, CH3), 0.87 (s, 9H, CH3), 1.04 and 1.56* (d, 3H, CH3, J 6.3 Hz), 1.06 and 1.08* (t, 3H, CH3, J 7.2 Hz), 1.38 and 1.43* (s, 3H, CH3), 2.40-2.50 (m, 2H, CH2), 2.88 (br s, 1H, H3') and 2.93* (m, 1H, H3'), 3.75* and 3.78 (s, 3H, OCH3), 4.03*(d, 1H, H2', J 2.0 Hz) and 4.25 (d, 1H, H2', J 2.0 Hz), 4.15* (m, 1H, H1") and 4.20 (dq, 1H, H1", J 2.8, 6.3 Hz), 5.90 (br s, 1H, NH). 13C NMR (125 MHz, CDCl3): -5.07, -4.40 (CH3), 7.99, 8.04 (CH3), 14.59, 16.86 (CH3), 17.91 ((CH3)3C-Si), 22.16, 22.78 (CH3), 25.72 (CH3), 32.46, 32.62 (CH2), 51.66, 52.74 (OCH3), 52.79, 53.03 (C2'), 59.63, 60.17 (C3'), 60.86, 60.92 (C2),64.27,65.28 (CHOTBS), 167.72, 167.97 (CONH), 171.41, 171.72 (CO2Me), 207.56, 207.83 (C=O). MS (ESI): m/z (I, %): 435 (100) [M+Na+MeCN]+, 394 (52) [M+Na]+, 372 (14) [M+H]+.
A. Major diastereoisomer 3: Sample of 90% purity was obtained by repeated column chromatography on the SiO2 of diastereomeric mixture 3 from the previous experiment. White crystals, mp. 68-70 oC, [αϕD -26.8o (c 1.00, CH2cl2)lH NMR (500 MHz, CDC13) δAAA: 0.05 (s, 6H, CH3), 0.87 (s, 9H, CH3),04 (d, 3H, CH3, J 6.4 Hz), 1.06 (t, 3H, CH3, J 7.2 Hz), 1.38 (s, 3H, CH3), 2.42-2.48 (m, 2H, CH2), 2.88 (t, 1H, H3', J 2.3 Hz), 3.78 (s, 3H, OCH3), 4.21 (dq, 1H, CH-OSi, J 2.3, J 6.3 Hz), 4.25 (d, 1H, H2', J 2.3 Hz), 5.80 (br. s, 1H, NH). 13C NMR (125 MHz, CDC13) 5 : -5.03, -4.34 (CH3), 8.09 (CH3), 14.61 (CH3), 17.96 ((CH3)3C-Si), 22.20 (CH3), 25.76 (CH3), 32.65 (CH2), 51.68 (OCH3), 52.81 (C2'), 59.65 (C3'), 60.90 (C2), 64.29 (CHOTBS), 168.01 (CONH), 171.76 (CO2Me), 207.90 (C=O).
II.(3S)-3-((1R)-1-{[tert-butyl(dimethyl)silyl]oxy} ethyl)azetidin-2-one 4: Rf 0.19 (petroleum ether - ethyl acetate, 7:3). -71.6o (c 1.00, CHC13).[α]ϕD IR, υ, cm-1: 3183, 2950, 1745, 1463,1372,1073.1H NMR (300 MHz, CDC13) δ: 0.07 (s, 6H, CH3), 0.87 (s, 9H, CH3), 1.20 (d, 3H, CH3, J 6.2 Hz), 3.22 (m, 1H, CHA), 3.29 (t, 1H, H3, J 5.1 Hz), 3.34 (m, 1H, CHB), 4.21 (m, 1H, HI'), 5.66 (br. s, 1H, NH). 13C NMR (125 MHz, CDC13) δ: δ;-5.05, --4.33 (CH3), 17.89 ((CH3)3C-Si), 22.48 (CH3), 25.72 (CH3), 37.62 (CH2), 59.22 (C3), 65.41 (CHOTBS), 169.61 (CONH).
Methyl 2-[(2S,3S)-3-((1R)-1-{[tert-butyl(dimethyl) silyl]oxy}ethyl)-1-(2-methoxy-2-oxoethyl)-4- oxoazetidin-2-yl]-2-(R,5)-methyl-3-oxopentanoate 5
Solution of NaHMDS (1 M solution in THF, 0.36 mL, 0.36 mmol) was added to a solution of compound 3 (0.1 g, 0.27 mmol) and methyl bromoacetate (0.07 g, 0.45 mmol) in 5 ml of anhydrous THF in an argon atmosphere at -78 °C. The reaction mixture was stirred for 1 h (TLC) at the same temperature, and was quenched by saturated aqueous NH4Cl solution (3 mL). THF was evaporated, the aqueous layer was extracted with ethyl acetate, the combined organic extract was washed with saturated brine, dried with MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, petroleum ether - ethyl acetate, 8:2) to afford lactam 5 (0.075 g, 60%) as oily liquid. Rf 0.30 (petroleum ether-ethyl acetate, 7:3). A mixture of C2-diastereomers in a 2:1 ratio. IR, υ, cm-1: 2955, 1768, 1751, 1714, 1437, 1257, 1208, 1114, 1076, 838, 778. *H NMR (500 MHz, CDC13) δ: 0.01, 0.015, 0.02 (s, 6H, CH3), 0.87 (s, 9H, CH3), 1.03 and 1.08* (t, 3H, CH3, J 7.2 Hz), 1.06 and 1.23* (d, 3H, CH3, J 6.1 Hz), 1.42* and 1.53 (s, 3H, CH3), 2.332.50 (m, 2H, CH2), 2.93 (d.d, 1H, H3', J 2.0, 3.7 Hz) and 2.97* (d.d, 1H, H3', J 1.2, 7.0 Hz), 3.67 (s, 3H, OCH3), 3.71* (s, 3H, OCH3), 3.75* (s, 3H, OCH3), 3.96* (d, 1H, HA, J 17.7 Hz) and 4.08 (d, 2H, HB, J 17.7 Hz), 4.12-4.20 (m, 1H, H1"), 4.25* and 4.42 (d, 1H, H2', J 2.0 Hz). 13C NMR (125 MHz, CDC13) δ: -4.58, -4.39 (CH3), 8.11, 8.25 (CH3), 14.39, 17.31 (CH3), 17.84, 17.87 ((CH3)3C-Si), 22.00, 22.74 (CH3), 25.76, 25.81 (CH3), 32.35, 32.43 (CH2), 42.63, 43.63 (CH2), 51.96, 52.14 (OCH3), 52.75, 52.83 (C2'), 57.22, 58.39 (OCH3), 59.20, 60.18 (C3'), 61.32, 61.43 (C2), 65.12, 66.83 (CHOTBS),168.27, 168.86 (CONH), 169.26, 171.28 (CO2Me), 207.63, 207.91 (C=O). MS (ESI): m/z (I, %): 466 (100) [M+Na]+, 507 (26) [M+Na+MeCN]+.
Dimethyl{[(2R,S,3Z)-2-((1R)-1-{[tert- butyl(dimethyl)silyl]-oxy}ethyl)-4-methyl-5-oxohept- 3-enoyl]amino}malonate 7
To a solution of the azetidinone 5 (0.06 g, 0.13 mmol) in anhydrous THF (4mL) was added NaHMDS (1 M in THF, 0.10 mL, 0.10 mmol) at -78 °C in argon atmosphere. The reaction mixture was stirred for 0.5 h (TLC) at the same temperature, and was quenched by saturated aqueous NH4Cl solution (3 mL).THF was evaporated, the aqueous layer was extracted with ethyl acetate, the combined organic extract was washed with saturated brine, dried with MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, petroleum ether - ethyl acetate, 8:2) to afford 7 (0.037 g, 62%) as 1:1 diastereomeric mixture of an oily liquid. Rf 0.30 ((petroleum ether - ethyl acetate, 7:3). IR, υ, cm-1: 3300, 2955, 1758, 1720, 1674, 1509, 1257, 1237, 1114, 978, 836, 777. H NMR (500 MHz, CDC13] <δ: 0.07, 0.08, 0.10 and 0.11 (s, 6H, CH3], 0. 88.and 0.09 (s, 9H, CH3], 1.10 (d.t, 6H, CH3, J 3.1, 7.3 Hz], 1.19 and 1.20 (d, 3H, CH3, J 6.3 Hz), 1.88 (t, 3H, CH3, J 1.5 Hz), 2.602.85 (m, 2H, CH2), 3.35 (m, 1H, H2') and 3.73 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 4.10 (m, 1H, CH-OSi), 5.25 and 5.27 (d, 1H, H1, J 4.1 Hz], 6.90 (m, 1H, H/], 7.40 (t, 1H, NH, J 7.4 Hz], 13C NMR (125 MHz, CDC13] δ : -5.03, -4.63 (CH3], 7.39, 7.44 (CH3], 13.01 (CH3],17.90 ((CH3]3C-Si], 20.43, 20.47 (CH3], 25.66 (CH3], 34.30, 34.50 (CH2), 51.87, 53.08 (OCH3), 52.96, 53.16 (C2'), 62.03, 62.12 (C2),69.90 (CHOTBS), 131.13 (C4), 135.86 and 135.90 (CH=), 166.49, 167.82 (CONH), 170.35 and 170.38 (CO2Me), 200.97 and 201.48 (C=O). MS (ESI): m/z (I, %): 444 (100) [MH]+.
Acknowledgment
The study was performed under financial support by the Russian Science Foundation (project no. 15-13-00 039).
References
- Garneau-Tsodikova S, Wright GD (2016) Med Chem Commun
- Worthington RG, Melander C (2013) Overcoming Resistance to β-Lactam Antibiotics. J Org Chem 78: 4207-4213.
- Berks AH (1996) Tetrahedron 52: 347-352;
- Berks AH (1996) Tetrahedron 52: 331.
- Basu MK, Banik BK (2001) Tetrahedron Lett 42: 187.
- Ham WH, Oh Ch Y, Lee YS, Jeong JH (2000) A New Synthesis of a Key Intermediate of β-Lactam Antibiotics via Diastereoselective Alkylation of β-Hydroxy Ester. J Org Chem 65: 8372.
- Nagahara T, Kanetani T (1987) Heterocycles 25: 729.
- Shiozaki M, Hiraoka T (1982) Tetrahedron 38: 3457; and references cited therein.
- Meyers AI, Sowin TJ, Schole S, Heda Y (1987) Tetrahedron Lett 28: 5103.
- Murayama T, Yoshida A, Kobayashi T, Miura T (1994) Tetrahedron Lett 35: 2271.
- Kagan HB (2003) Tetrahedron 59: 10351.
- Krief A, Laval AM (1999) Coupling of Organic Halides with Carbonyl Compounds Promoted by SmI2, the Kagan Reagent. Chem Rev 99: 745.
- Molander GA, Harris CR (1996) Sequencing Reactions with Samarium (II) Iodide. Chem Rev 96: 307.
- Nicolaou KC, Ellery SP (2009) Samarium Diiodide Mediated Reactions in Total Synthesis. Chen JS Angew Chem Int Ed Engl 48: 7140.
- Utimoto K, Takai T, Matsui T, Matsubara S (1997) Bull Soc Chim Fr 134: 365.
- Utimoto K, Matsui T, Takai T, Matsubara S (1995) A Preparation of β-Oxoester Enolate Equivalents from SmI2 and α-Bromoalkanoates. Chem Lett 24: 197.
- Castagner B, Lacombe P, Ruel R (1998) Samarium(II) Iodide-Mediated Synthesis of Chlorohydrins. J Org Chem 63: 4551.
- Balaux E, Ruel R (1996) Tetrahedron Lett 37: 801.






























