Pharmaceutical and Biological Effects of Suramin and Potential of Its Derivatives and Analogues
Shwu-Chen Tsay1*, Nitesh K. Gupta1, Sergey O Bachurin2 and Jih Ru Hwu1*
1Department of Chemistry, National Tsing Hua University, Taiwan
2Department of Medical and Biological Chemistry, Institute of Physiologically Active Compounds, Russian Academy of Science, Russia
Submission: May 12, 2017; Published: June 13, 2017
*Corresponding author: Shwu-Chen Tsay and Jih Ru Hwu, Department of Chemistry & Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing Hua University, Hsinchu 30013, Taiwan, Tel: +886 35 725 813; Email: tsay.susan@gmail.com; jrhwu@mx.nthu.edu.tw
How to cite this article: Shwu Chen Tsay*, Nitesh K Gupta, Sergey O Bachurin and Jih Ru Hwu* Pharmaceutical and Biological Effects of Suramin and Potential of Its Derivatives and Analogues. Organic & Medicinal Chem IJ. 2017; 2(4): 555593. DOI: 10.19080/omcij.2017.02.555593
Abstract
As a popular drug, suramin has attracted much attention for a century. It has an extensive record of clinical trials. This review article gives a brief introduction of its wide biological roles, remarkable pharmacological activities, and several side effects. Chemists and biological scientists have been working together for the design and synthesis of its derivatives and analogues. Some structure-activity relationships have been realized and provide guidelines for its prosperous development in the near future.
Keywords: Suramin; Derivative; Analogue; Synthesis; Anticancer; Antiviral; Antiparasitic; Enzyme inhibition
Mini Review
Suramin (1) is a synthetic compound and has been used as a medication for a long period of time. As early as a hundred years ago (i.e., 1916), Oskar Dressel and Richard Kothe of Bayer, Germany developed this organic compound in success [1]. For commercial reasons, Bayer kept its structure as a secret. Till1924, a French medicinal chemist Ernest Fourneau and his team of the Pasteur Institute elucidated and published its formula[1].
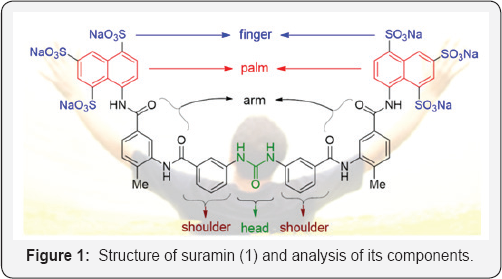
As a symmetric molecule with the formula C51H40N6023S6, suramin has an NH-CO-NH functionality in the center as the “head” (Figure 1). Starting from the head as a joint, the molecule is built by two benzene rings with amide linkers on both flanks as the “shoulders,” two amidotoluene as the “arms”, two naphthalene rings as the “palms,” and then six sulfonate groups as the “fingers”. Many medical applications are associated with this symmetric compound [2].
Suramin is used as a drug for the treatment of human sleeping sickness caused by trypanosomes [3]. Medical, biological, and chemical scientists have been researching into its further potential applications. In 1984, Broder, et al. reported that this drug acts as an inhibitor of HIV-1 [4]. In 1985, Rozenbaum and co-workers reported equally promising anti-HIV activity with a polyoxometalate in the treatment of HIV infection [5]. In 2012, Bolognesi et, al. [6] unveiled the activity of suramin against RNA viruses. Their approaches are structure-based for inhibition of norovirus RNA dependent RNA polymerases. In 2013, De Clercq [7] published a nice review on his personal perspective on the drug development, in which suramin is a topic as a potent inhibitor of the reverse transcriptase. It could be due to the hexasulfonate feature (i.e., the six “fingers”) [8]. In 2015, van Hermert, et al. [9] reported the anti-parasitic drug suramin inhibits the replication of chikungunya virus (CHIKV) and other alphaviruses. While being a potent inhibitor of RNA synthesis in vitro, suramin mainly inhibits an earlier, post-attachment step of the CHIKV replicative cycle, likely viral entry in cell culture. It appears to inhibit (re)initiation of CHIKV RNA synthesis, by possible interfering with binding of the template RNA. In the same year, Lin, et al. [10] reported that suramin is able to reduce viral release and cell-to-cell transmission of CHIKV. It possesses potential as a novel anti-CHIKV agent targeting viral entry and extracellular transmission. Then Lin, et al. [11] evaluated the anti-CHIKV activities of suramin in vivo in terms of histopathology, viral burden, and disease score. They found that suramin treatment reduces chikungunya pathogenesis in mice. In addition to the CHIKV, Altmeyer, et al. [12] found that the approved pediatric drug suramin can inhibit enterovirus 71 infections by neutralizing viral particles prior to cell attachment. Consequently, it was identified as a clinical candidate for further development as a therapeutic or prophylactic treatment for severe enterovirus 71 disease.
Recently, Katanaev, et al. [13] has commented on suramin in his review article that this drug demonstrates a dose-dependent anti-proliferative effect in many human cancer cell lines. Its anticancer effects are due to the inhibition of binding of growth factors (e.g., vascular endothelial growth factor) to their cognate receptors, the folate metabolism, the WNT signaling pathways resulting in its complete blockade. Furthermore, fibroblast growth factors (FGFs) play crucial roles in the regulation of key cellular processes such as angiogenesis, differentiation, and tumor growth. Suramin is used as a potent inhibitor of FGF- induced angiogenesis. On the basis of the results of Yu, et al. [14], a model was proposed to explain the molecular mechanism(s) underlying the antimitogenic activity of suramin. It is the first time that interaction sites of suramin on FGF are characterized. Therefore it shows promise for its prospects against cancers of various types.
Moreover, suramin is among one of the most studied selective histone deacetylase (HDAC) inhibitors as reported by Jiang, et al. [15]. The roles of HDAC inhibitors in Alzheimer's disease possess two aspects. First, HDAC inhibitors constrain A^-induced hyperphosphorylation of tau protein. Second, HDAC inhibitors alter the expression of important genes that participate in the learning and memory. HDAC inhibitors are suggested to function as neuroprotectors by enhancing synaptic plasticity and learning and memory in a wide range of neurodegenerative and psychiatric disorders [15]. Examples include Alzheimer's disease and Parkinson's disease.
Suramin is also known as an antipurinergic therapy (APT) mediator [16]. It can trigger the mechanism linked to mitochondria and influence immunity. Suramin has the ability to stabilize cell-to-cell signaling, locomotor function and coordination, normalize brain synapse structure, and social behavior. Additionally, suramin could provide new therapeutic windows for Autism spectrum disorder (ASD) subtypes. A number of clinical studies have explored the scope of APT with suramin to assist the genetic, metabolic, and environmental risk factors of ASD.
The application of this molecule was abandoned for further clinical treatment to HIV/AIDS patients because of a significant number of fatal occurrences [17]. This drug was found too toxic towards liver and kidney. Due to some side effects emerging [18], suramin is considered second-line treatment for early-stage disease. These effects include diarrhea, headache, nausea, skin tingling, vomiting, and weakness.
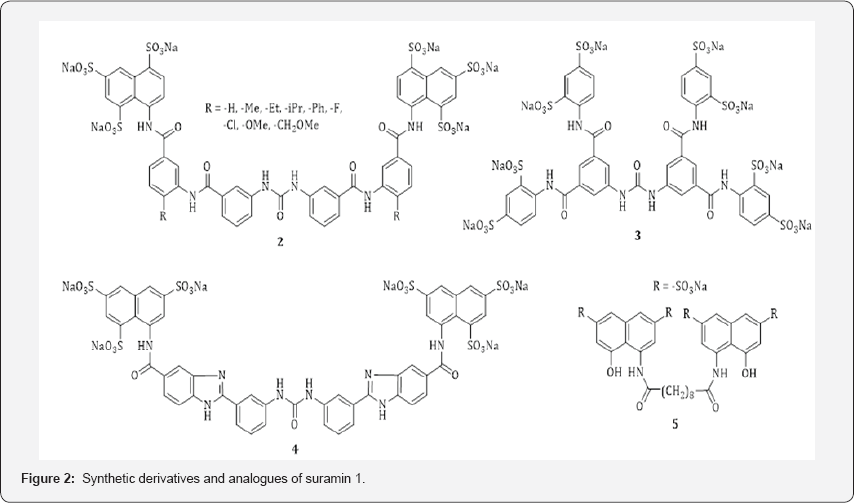
Some suramin derivatives and analogues have been synthesized and their biological roles have been evaluated. Representative examples include the replacement of the methyl groups of suramin by alkyl, aryl, fluorine, chlorine, methoxy, and methoxymethyl substituents (i.e., 2) as reported by Kassack, et al in 2005. [19] (Figure 2). They also synthesized a series of analogues (e.g., 3) of suramin [20]. These derivatives and analogues were tested at P2Yn, P2Yj, and P2Y2 receptors antagonist and their structure-activity relationship were illustrated. In 1998, Kreimeyer, et al. [21] described the synthesis and anti-HIV activity of suramin analogues bearing a 2-phenylbenzimidazole moiety (i.e., 4 in Figure 2). Compounds with a skeleton of that kind showed an increased rigidity. In 1992, Mohan, et al. [22] synthesized several naphthalenedi- and trisulfonic acids (i.e., 5 in Figure 2) and evaluated these analogues of suramin for inhibitory potential against cytopathogenesis and purified recombinant human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) reverse transcriptase.

Bolongnesi et al. [23] designed and studied novel suramin derivatives. Through collaboration with synthetic chemists, they synthesized a modified suramin molecule 7 and presented the crystal structure analyses of NV-RdRps in complexes with a suramin fragment 6, and evaluated inhibitory parameters for five related compounds targeting mNV and hNV-RdRps (Figure 3) [23]. In comparison with suramin (1) bearing six hydrophilic sulphonate groups, the new and symmetric compound 7 containing four sulphonate groups exhibits the same potency as suramin against NV-RdRps.
Some suramin derivatives and analogues obtained so far by chemical synthesis may not be more effective than the parent compound. Nevertheless, results from the study of their activities provide insight into the structural features that are of importance for inhibitory activities of the parent suramin molecule observed in vitro and in cell culture.
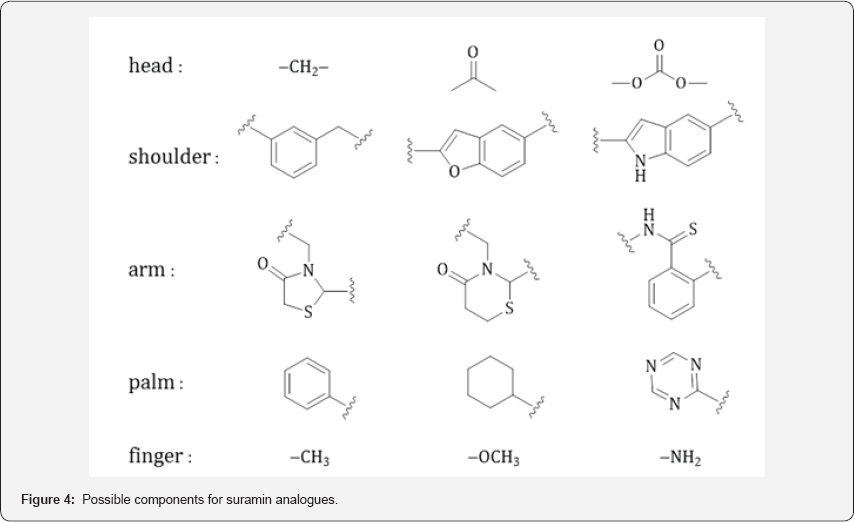
A logic approach for future synthetic works includes replacement of the essential moieties with various or even pharmacophores [24]. The possible variations of choices are listed in the Figure 4. The “head” of carbamide in suramin could be replaced by methylene, carbonyl, and carbonate joints. The “shoulders” of benzene rings with amide linkers could be replaced by benzyl, benzofuran, and indole nuclei. The “arms” of amidotoluene could be replaced by a thiazolidinone, thiazinanone, and thiobenzamide moieties. The “palms” of naphthalene rings could be replaced by a benzene, cyclohexane, and 1,3,5-triazine rings. The “fingers” of sulfonate groups could be replaced by methyl, methoxy, and amino groups. For example, the pharmacological components of suramin and its derivatives and analogues may allow binding against purinergic receptors without release. Accordingly, new compounds may serve as an antagonist of extracellular APT, and other nucleotides. The development of suramin derivatives and analogues offer a different and promising solution to the diseases faced by the current societies.
Acknowledgement
For financial support, we thank Ministry of Science and Technology (grant No. MOST 103-2113-M-007-018-MY3) and Ministry of Education of R.O.C. (grant Nos. 104N2011E1 and 104N2016E1)
References
- Sneader W (2005) Drugs Originating from the Screening of Dyes. In: Sneader W, Drug Discovery: A History. John Wiley, Chichester, West Sussex, England pp: 378-379.
- Babokhov P, Sanyaolu A O, Oyibo W A, Fagbenro-Beyioku A F, Iriemenam N C (2013) A Current Analysis of Chemotherapy Strategies for the Treatment of Human African Trypanosomiasis. Pathog Glob Health 107(5): 242-252.
- WHO Model List of Essential Medicines. 18th list (April 2013) World Health Organization. October 2013. Retrieved 4 May 2017.
- Mitsuya H, Popovic M, Yarchoan R, Matsushita S, Gallo R C, et al. (1984) Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III. Science 226: 172-174.
- Rozenbaum W, Dormont D, Spire B, Vilmer E, Gentilini M, et al. (1985) Antimoniotungstate (HPA 23) Treatment of Three Patients with AIDS and One with Prodrome. Lancet 325(8426): 450-451.
- Mastrangelo E, Pezzullo M, Tarantino D, Petazzi R, Germani F, et al. (2012) Structure-Based Inhibition of Norovirus RNA-Dependent RNA Polymerases. J Mol Biol 419: 198-210.
- De Clercq E (2013) Antiviral Drug Development- Success and Failure: A Personal Perspective with a Japanese Connection. Antivir Chem Chemother 23: 45-55.
- De Clercq E (1979) Suramin: a Potent Inhibitor of the Reverse Transcriptase of RNA Tumor Viruses. Cancer Lett 8: 9-22.
- Albulescu I C, van Hoolwerff M, Wolters L A, Bottaro E, Nastruzzi C, et al. (2015) Suramin Inhibits Chikungunya Virus Replication through Multiple Mechanisms. Antiviral Res 121: 39-46.
- Ho Y J, Wang Y M, Lu J W, Wu T Y, Lin LI et al. (2015) Suramin Inhibits Chikungunya Virus Entry and Transmission. PLoS ONE 10(7): e0133511.
- Kuo S C, Wang Y M, Ho Y J, Chang T Y, Lai Z Z, et al. (2016) Suramin Treatment Reduces Chikungunya Pathogenesis in Mice. Antiviral Res 134: 89-96.
- Ren P, Zou G, Bailly B, Xu S, Zeng M, et al. (2014) The Approved Pediatric Drug Suramin Identified as a Clinical Candidate For The Treatment of EV71 Infection-Suramin Inhibits EV71 Infection in vitro and in vivo. Emerg Microbes Infect 3: e62.
- Ahmed K, Shaw H V, Koval A, Katanaev V L (2016) A Second WNT for Old Drugs: Drug Repositioning Against WNT-Dependent Cancers. Cancers 8: 66.
- Kathir K M, Kumar T K S, Yu C (2006) Understanding the Mechanism of the Antimitogenic Activity of Suramin. Biochemistry 45: 899-906.
- Xu K, Dai X L, Huang H C, Jiang Z F (2011) Targeting HDACs: A Promising Therapy for Alzheimer's Disease. Oxid Med Cell Longev 2011: 143269.
- Hamidpour R, Hamidpour S, Hamidpour M, Zarabi M, Sohraby M, et al. (2016) Antipurinergic Therapy with Suramin as a Treatment for Autism Spectrum Disorder. J Biomed Sci 5(2): 1-7.
- Kaplan L D, Wolfe P R, Volberding P A, Feorino P, Abrams D I, et al. (1987) Lack of Response to Suramin in Patients with AIDS and AIDS- Related Complex. Am J Med 82(3): 615-620.
- Kappagoda S, Singh U, Blackburn B G (2011) Antiparasitic Therapy. Mayo Clin Proc 86(6): 561-583.
- Ullmann H, Meis S, Hongwiset D, Marzian C, Wiese M, et al. (2005) Synthesis and Structure-Activity Relationships of Suramin-Derived P2Y11 Receptor Antagonists with Nanomolar Potency. J Med Chem 48(22): 7040-7048.
- Kassack M U, Braun K, Ganso M, Ullmann H, Nickel P, et al. (2004) Structure-Activity Relationships of Analogues of NF449 Confirm NF449 as The Most Potent and Selective Known P2X1 Receptor Antagonist. Eur J Med Chem 39: 345-357.
- Kreimeyer A, Muller G, Kassack M, Nickel P, Gagliardi A R T (1998) Suramin Analogues with a 2- Phenylbenzimidazole Moiety as Partial Structure; Potential anti HIV- and Angiostatic Drugs, 2J): Sulfanilic Acid-, Benzenedisulfonic Acid-, and Naphthalenetrisulfonic Acid Analogues. Arch Pharm Pharm Med Chem 331: 97-103.
- Tan G T, Wickramasinghe A, Verma S, Singh R, Hughes S H, et al. (1992) Potential Anti-AIDS Naphthalenesulfonic Acid Derivatives. Synthesis and Inhibition of HIV-1 Induced Cytopathogenesis and HIV-1 and HIV-2 Reverse Transcriptase Activities. J Med Chem 35: 4846-4853.
- Croci R, Pezzullo M, Tarantino D, Milani M, Tsay SC, et al. (2014) Structural Bases of Norovirus RNA Dependent RNA Polymerase Inhibition by Novel Suramin-Related Compounds. PLoS ONE 9: e91765.
- Khanam H, Shamsuzzaman (2015) Bioactive Benzofuran Derivatives: A Review. Eur J Med Chem 97: 483-504.






























