Medicinal Applications, Phytochemistry and Pharmacology of Hymenodictyon excelsum (Roxb.) Wall: A Review
Paramita Chakraborty, Sajeesha Sasi, Anuja A Nair, Nishat Anjum and YC Tripathi*
Chemistry Division, Forest Research Institute, Dehradun, India
Submission: April 15, 2017; Published: May 05, 2017
*Corresponding author: YC Tripathi, Chemistry Division, Forest Research Institute, P.O. New Forest, Dehradun - 248006 India, Tel: +0135-2224207: +91-9412050775; Email: tripathiyc@gmail.com
How to cite this article: Paramita C, Sajeesha S, Anuja A Nair, Nishat A, YC Tripathi. Medicinal Applications, Phytochemistry and Pharmacology of Hymenodictyon excelsum (Roxb) Wall: A Review. Organic & Medicinal Chem I J. 2017; 2(3): 555589.DOI:10.19080/OMCIJ.2017.02.555589
Abstract
Hymenodictyon excelsum Roxb. Wall (Rubiaceae) has been used in traditional medicine for a wide range of ailments related to digestive, endocrine, reproductive, and respiratory systems. Additionally, it is also used in gastrointestinal tract and urinary tract infection. This review gathers the fragmented information available in the literature regarding morphology, ethnomedicinal applications, phytochemistry, and pharmacology of H. excelsum. Relevant information on H. excelsum was compiled from electronic databases such as Academic Journals, Ethnobotany, Google Scholar, PubMed, Science Direct, Web of Science, and library search. Worldwide ethnomedical uses of H. excelsum are recorded which have been traditionally practiced for the treatment of different types of health disorders. Phytochemical research have led to isolation and characterization of different types of bioactive compounds in H. excelsum and pharmacological studies have shown some promising pharmacological activities. H. excelsum has emerged as a good source of traditional medicine for the treatment of various ailments. It is a promising candidate in pharmaceutical biology for the development/ formulation of new drugs and future clinical uses.
Keywords: Hymenodictyon excelsum; Traditional medicinal uses; Phytochemistry; Pharmacology
Introduction
Hymenodictyon is a genus of flowering plants in the family Rubiaceae comprising of about 30 species. Hymenodictyon was named by Nathaniel Wallich in 1824 in an addendum to William Roxburgh's Flora Indica, an edition published by Carey and Wallich after Roxburgh's death. The generic name is derived from two Greek words, hymen, 'membrane', and diktyon, 'net'. It refers to the wing that surrounds each seed. Molecular phylogenetic studies have shown that Hymenodictyon is paraphyletic over the Madagascan genus Paracorynanthe. In Hymenodictyon and Paracorynanthe, the stipules bear large deciduous glands called colleters. The corolla tube is narrow at the base, gradually widening toward the apex. The fruit is a woody capsule. The species belonging to this genus are having oppositely arranged serrated leaves, small, clustered flowers and many seeded capsules. The genus comprises trees and shrubs distributed mostly in tropical and sub-tropical parts of Asia and Africa [1]. Some of the species are commercially useful for tanning and dyeing purposes while remaining are only useful for the timber wood.
Hymenodictyon excelsum Roxb. Wall. syn H. orixense Roxb. Mobb. belonging to the family Rubiaceae and commonly known as Bhorsal, Kukurkat, Bhaulan, Bauranga, Pottaka, Kusan, Kadambu (India), Kuthan (Burma), and Lala (Thailand), is a medium to tall growing, deciduous tree, 10-12 m in height, usually with a straight cylindrical bole, a rounded crown, grey-brown-tinged bark, oblong, ovate, or elliptic, glabrous green leaves, greenish- white, fragrant flowers and ellipsoid capsules containing winged seeds. Bark is mostly furrowed and rough, 10-20 cm thick, exfoliating in irregularly shaped, softish scales. Stipules are linear to lanceolate or ovate to lanceolate, c. 15 mm long, apex acuminate, deciduous, pubescent. Leaves are deciduous; petioles 10-60 mm long, green-white-tinged, puberulous; blades ovate, elliptic to lanceolate or oblong to lanceolate, pale green above, lightgreen-tinged beneath, glabrous to puberulous above, pubescent to puberulous beneath, membranaceous, apex acuminate, base acute to cuneate; margins glabrous, ciliate; midribs drying yellow-red-tinged, sometimes brown-black- tinged, puberulous above, puberulous to pubescent beneath; secondary veins seven to ten pairs per side, colour unknown, inconspicuous above, conspicuous beneath, glabrous; without domatia.
Leaves are ovate-elliptic or almost rounded 10-24 cm long, 7-12.5 cm wide, pointed at both ends, and hairy on both surfaces. Flowers whitish or yellowish green are stalked, fragrant, about 0.5 cm long, and borne in terminal, drooping panicles. Capsule is oblong-elliptic; Corolla-tube is slender and 5-lobed. Fruit, a capsule, is ellipsoid, 2 to 2.5 cm long, growing on recurved, thick pedicels 5 to 12 mm long. Seeds are many, flat, winged all around the margin, about 1 cm long, including the wing [2-4]. The wood of H. excelsum is soft and has limited use, mostly for boxes. The wood is used as planks in building houses and boats; for making boxes, packing cases, pencils, toys model making and matches [5]. In India, it is used as a cheaper grade of wood for making furniture, wrapper bobbins and wool boards. The bark obtained is useful for tanning purposes while leaves are useful for dyeing and as fodder for cattle [6].
Distribution
H. excelsum is mainly found in secondary forests at low altitudes, often about cliffs near the sea. It is distributed throughout Oceania and Southeast Asia. It occurs in Nepal, Burma, Java Bangladesh, Cambodia, Indonesia, Malaysia, Thailand, Philippines, Vietnam and Philippines. It is distributed throughout India, and in the Himalayan region of India [7-8] except Jammu and Kashimi [2].
Traditional Medicinal Uses
Primarily bark and leaves of H. excelsum have been used in traditional medicine systems across the world for treatment of various ailments. bark exhibits wide spectrum of medicinal value. Various plant parts reported to be used in burning sensation in chest, emaciation, and carbuncle and useful in fever, sores, smallpox, atrophy and lactation complaints. It also increases taste and appetite. Decoction used in diarrhoea [9-10]. In traditional medicine system bark and leaves of the plant are attributed to various medicinal properties. Bark has been used as an astringent and febrifuge and for treatment of fever and tumours, while the leaves are used to treat ulcers, sialitis, sore throat, tonsillitis and inflammatory conditions [11-12]. The Inner bark of the plant is traditionally used as febrifuge, astringent and antiperiodic, especially for tertian ague. It is also used as a substitute for quinine and for night blindness. The crushed and powdered bark is orally used for treating hemorrhoids (piles). According to Ayurveda, bark is hot, pungent, bitter, increases taste and appetizer. It is good for throat and cures all tumours. In India, the bitter bark is used as astringent and febrifuge; root, wood and stem-bark used as for fevers and to relieve thirst. It is also known for its wound healing property [13-14]. As antiperiodic, bark used as substitute for cinchona bark. Inner bark is bitter; outer layer of the bark is tasteless. Its bark is used as an astringent and febrifuge, while its leaves are used to treat jaundice, fever, ulcer, sialitis, sore throat, tonsillitis, and inflammation [15]. Powder of the root is given with cow's milk in bodily inflammation. Bark is used as powder to kill tapeworms and to cure dysentery [16].
Phytochemistry
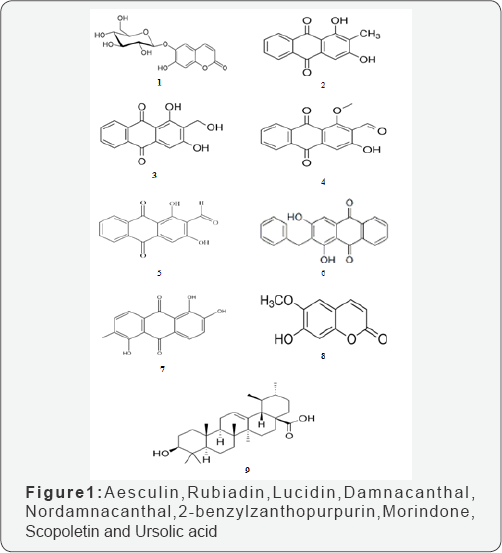
H. excelsumhas been investigated for its considerable number of important phytoconstituents [17-22]. The chemical constituents previously reported to be found in this plant were coumarins [23] and anthraquinones [24]. The stem bark contains tannin, toxic alkaloid hymenodictine, a bitter substance, aesculin(l), an apioglucoside of scopoletin, hymexelsin [10,19]. Anthraquinones, rubiadin(2) and its methyl ether, lucidin (3), damnacanthal (4), nordamnacanthal(5), 2-benzylzanthopurpurin(6), anthragallol, soranjidol and morindone(7) have also been isolated from roots [4]. Hymenodictyoline obtained from H. excelsum is one of the few alkaloids which do not contain oxygen. Aesculin (β-methylaesinietin), scopoletin(8), hymenodictyonim (a toxic alkaloids); alanine, arginine, cystine, glycine, leucine, fruitore, galactose, glucose from barks; anthragallol, 6-methyalizarin, subiadin and its 1-methylester, soranjidol, isolated from roots [12]. Studies have also reported acetylenic fatty acids, a new triglyceride, and 11 known compounds, among them, ursolic acid(9), oleaqnolic acid, uncarinic acid E, β-sitosterol [23]. The roots of H. excelsum also reported to contain anthragallol, 6-methylalizarin, soranjidiol, morindone and triterpenes including 3β-hydroxy-11-oxours-12-en-28-oic acid; 3β-hydroxy- 27-p-(Z)- coumaroyloxyolean-12-en-28-oic acid; 3-oxo-11α,12α- epoxyurs-13p,28-olide; 3β-hydroxy-11α,12α-epoxyurs-13β,28- olide; 3β-hydroxyurs-11-en-13(28)-lactone; oleanolic acid; uncarinic acid E (3β-hydroxy-27-(E)-p-coumaroyloxyolean- 12-en-28-oic acid, 3β-(formyloxy)-urs-12-en-28-oic acid [25] (Figure 1).
Pharmacology
The plant and some of its active chemical constituents have been investigated for various pharmacogical properties. The leaves and bark of the plant have been reported to have antimicrobial, anticoagulant, anti-inflammatory [26] and sun- screening activity [27]. Furthermore, antioxidant, antimicrobial, anti-inflammatory, analgesic, depressing, antipyretic, atherothrombolytic and moderately antiamylase activities of H. excelsum extracts have been reported [28]. A precise description of various pharmacological activities is derailed hereunder.
Antimicrobial Activity
H. excelsum bark exhibited a bactericidal effect against S. aureus at a concentration of 500µg/ml. The acetone extract 100mg/ml showed inhibition zone of 24mm against Pseudomonas aureginosa which is more than control assuring a good antibacterial activity against the bacterium [29]. Methanolic bark extract of H. excelsum also showed antimicrobial evidence against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Mycobacterium smegmatis and Candida albicans and the leaves for antiinflammatory activity [30]. Inhibitory effect of the methanolic extract of H. excelsum bark assessed against some important and frequently occurring pathogenic fungi viz., Alternaria alternate, Aspergillus flavus, Cladosporium cladosporidies, Drechslera halodes and Fusarium moniliforme by agar-well diffusion method showed significant antifungal the bark extract in a dose dependant manner. The extract at concentration of 40mg/ml showed growth inhibition almost at par with synthetic fungicide, Carbendazim [31].
Anti-inflammatory Activity
In vitro anti-inflammatory activity studied using inhibition of protein denaturation and human red blood cell (HRBC) membrane stabilization methods. Inhibition of protein denaturation was found to be 82.64 ± 0.6 % at a dose of 250 µg/ml that indicated remarkable in vitro anti inflammatory activity [32]. Methanol extract of H. excelsum bark reported to exhibit significant antiinflammatory activity tested by egg albumin induced rat paw edema model and found comparable to the standard antiinflammatory drug Piroxicam [33]. Hydro alcoholic extract of leaves of the plant evaluated for anti-inflammatory activity by carrageenan induced oedema method. The percentage inhibition of oedema at 4th hour of the dose 200mg/kg was 42.62%, and the dose 400mg/kg was 62.63%, the standard 61.78% [34].
Antioxidant Activity
Methanol extract of H. excelsum bark showed effective free radical scavenging at higher concentrations [31,35].
Antimalarial activity
The ethyl acetate extract of H. excelsum has shown dose dependent percentage inhibition of schizont maturation of Plasmodium falciparum, hence possess significant in vitro and in vivo antimalarial activity [36].
Anticoagulant activity
The chemical constituents of H. excelsum named scopoletin and its galactoside (each 25 mg/kg) reported to show slight increase in prothrombin time however, higher doses (100 mg/ kg for the former, 200 mg for the latter) enhanced prothrombine time significantly suggesting their promising anticoagulant activity [27].
Antitumour Activity
H. excelsum is traditionally used for treatment of tumour as it contains phytochemicals of anthraquinone and coumarin group. The structure-activity relationship of coumarin and its derivatives has been studied and ascertained by semi empirical molecular orbital method [37].
Cytotoxic activity
Cytotoxic activity of H. excelsum bark extract was investigated against healthy mouse fibroblasts (NIH3T3), healthy monkey kidney (VERO) and four human cancer cell lines (gastric, AGS; colon, HT-29; and breast, MCF-7 and MDAMB-231) using MTT assay. High cytotoxicity across all cell lines tested was exhibited by bark extract. H. excelsum bark showed strong but non-selective cytotoxicity that support for the traditional use of the 'active' plant as anticancer agents [22]. Cytotoxic activity of methanolic extract of the bark of H. excelsum on DLA cells by trepan blue dye exclusion method and observed 100% cytotoxicity at 200|ig/ml [30]. The anthraquinone and coumarin principles of H. excelsum have an anti-prostate cancer effect that has been proposed to be exerted by antagonistic effects on human androgen receptor [38]. Anti-proliferative activity of stem bark of the plant evaluated using the Ehrlich As cites Carcinoma (EAC) cells on Swiss albino mice have showed significant decrease in tumour volume, viable cell count, tumour weight and elevated the life span of EAC tumour bearing mice. Haematological profile such as RBC, haemoglobin, WBC and lymphocyte count reverted to normal level in treated mice. This revealed that the extract has potent dose dependent anticancer activity that is comparable to that of 5-fluorouracil [34].
Conclusion
An extensive literature survey revealed that H. excelsum is an important medicinal plant used for the ethnomedicinal treatment of fever, hemorrhoids, inflammation, abdominal disorders, infectious diseases, and many more throughout the world. Pharmacological studies carried out on the crude extracts, and individual chemical constituents of H. excelsum provide an experimental support for its various traditional medicinal uses. Recent studies have been focused on evaluating the antimicrobial (antibacterial), antifungal, anti-inflammatory, cytotoxicity, antioxidant activities. Most of the mentioned pharmacological studies were aimed at validating its traditional uses. It has been found that some of its traditional uses have been extensively explored by several research groups, like anti-inflammatory and antimicrobial activities. However, adequate experimental evidence are lacking to substantiate its various other traditional therapeutic applications that need further scientific validation.
Primarily bark and leaves of H. excelsum have been reported to be employed in the treatment of some ailments in varied geographical locations. The explanation for such a practice warrants further phytochemical and pharmacological studies. Furtherefore, majority of the pharmacological studies that have been done with crude extracts of various parts of the plant. Thus, it is difficult to reproduce the outcomes of these studies and to locate the precise bioactive metabolite. Hence, there is a need for phytochemical standardization and identification of further bioactive candidates. Phytochemical research carried out on H. excelsum has led to the isolation of few classes of plant metabolites. However, the vast traditional use and proven pharmacological activities indicate that an immense scope still exists for its phytochemical exploration. However, the potent bioactive secondary metabolite with potential for pharmacological effects e.g. anticancer, scavenging activity, etc. as described by earlier researchers. Therefore, there is a vast scope for establishing a relation between given phytoconstituents and biological activities. The outcome of the future research in the above-mentioned areas will provide a convincing support for the future clinical uses of H. excelsum in modern medicine.
References
- Sylvain GR, Birgitta B (2006) Taxonomic revision of the tribe Hymenodictyeae (Rubiaceae, Cinchonoideae). Botanical Journal of the Linnean Society 152: 331-386.
- Deb DB (1989) Taxonomic revision of the genus Hymenodictyon (Rubiaceae). Journal of Economic Taxonomy and Botany 13: 673-682.
- Asolkar LV, Kakkar KK, Chakra OJ (1992) Glossary of Indian Medicinal Plants with Active Principles (Second Supplement) Part-1 (A-K) 19651981. NISC, CSIR, New Delhi, India.
- Rastogi RP, Mehrotra BN (1993) Compendium of Indian medicinal plants, Vol. 3, edited by Rastogi R.P. Central Drug Research Institute and Publications & Information Directorate New Delhi.
- Purkayastha SK (1996) A manual of Indian timbers. Calcutta, Sribhumi publishing Co pp: 407-409.
- The Wealth of India (1959) Council of Scientific and Industrial Research, New Delhi pp: 149- 151.
- Gurung AA (2002) study on medicinal plant and their traditional uses in Chitre Parbat and Bhadaure/Tamagi Kaski, West Nepal. Central Department of Botany, Tribhuvan University pp 80.
- Razafimandimbison SG, Bermer B (2006) Taxonomic revision of the tribe Hymenodictyeae (Rubiaceae, Cinchonoideae). Bot J Linn Soc 152: 331-386.
- Sarin YK (196) Illustrated manual of Herbal Drugs used in Ayurveda, Council of Scientific and industrial Research and Indian council of Medicinal Research New Delhi, India.
- Ghani A (2003) Medicinal plants of Bangladesh with chemical constituents and uses, 2nd edn, Asiatic Society of Bangladesh, Dhaka.
- Rai LK, Sharma E (1994) Medicinal Plants of the Sikkim Himalaya. Prishen Singh, Mahendra Pal Singh, Dehradun pp: 48.
- Chatterjee A, Pakrashi SC (1997) The Treatise on Indian Medicinal Plants (Vol. 5). National Institute of Science Communication, New Delhi pp: 84- 85.
- Prashant YM, Vijay VB (2011) Ethnomedicinal wisdom of tribals of Aurangabad district (MS), India. Ind J Nat Prod Resour 2(1): 102-109.
- Abhijit D, Jitendranath DE (2011) Traditional use of medicinal plants in paediatric and maternal care practiced by the ethnic groups of Purulia district, west Bengal, India. Int J Med Arom Plants 1: 189-194.
- Bandaranayake WM (1998) Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 2: 133-148.
- Maitreya BB (2015) An overview of Ethnomedicinal plants of Family Rubiaceae from Sabarmati River of Gujarat state, India. Int J Pharm Life Sci 6(5): 4476-4480.
- Stanley GC, Lionel SJ (1916) The constituents of the bark of the Hymenodictyon excelsum. J Proc Asiatic Soc Bengal 12: 161.
- Stanley GC, Simonsen J, Lionel SJ (1918) The constituents of the bark of the Hymenodictyon excelsum. J Chem Soc 114(1): 151-152.
- Rao PS, Asheervadam Y, Khaleelullah M, Subba RN, Murray RDH (1988) Hymexelism an apiose-containing scopoletin glycoside from the stem bark of Hymenodictyon excelsum. J Nat Prod 51(5): 959- 961.
- Joshi SN, Baxi AJ (1990) Free amino acid and sugar content of bark of Hymenodictyon excelsum Wall. J Institut Chem (India) 62(5): 206.
- Joshi SN, Baxi AJ (1993) Lipids of the bark of Hymenodictyon excelsum Wall. J. Institut. Chemists (India) 65(2): 57.
- Akter R, Shaikh JU, Darren GI, Evelin T (2014) Cytotoxic activity screening of Bangladeshi medicinal plant extracts. J Nat Med 68(1): 246-252.
- Parichat N, Wichan K, Decha L, Nuanchawee W, Chudaporn P, et al. (2009) Acetylenic fatty acids, triglyceride and triterpenes from the leaves of Hymenodictyon excelsum. Chem Pharmaceut Bull 57(8): 860862.
- Brew EJC, Thomason RH (1971) Naturally occurring quinones. Part XIX. (1971), anthraquinones in Hymenodictyon excelsum and Damnacanthus major. J Chem Soc C.
- Nareeboon P, Komkhunthot W, Lekcharoen D, Wetprasit N, Piriyapolsart C, et al. (2009) Acetylenic fatty acids, triglyceride and triterpenes from the leaves of Hymenodictyon excelsum. Chem Pharm Bull (Tokyo) 57(8): 860-862.
- Chandur Uma, Rao BG, Mallikarjun Rao T, Killari K (2013) Evaluation of the anti-inflammatory activity of the leaves of Hymenodictyon excelsum Wall. Biochem & Pharmacol 2: 4.
- Jagdish Prasad P, Subba Rao N (1988) Anticoagulant and Antinflammatory and Sun-screening effects of Hymenodictyon excelsum. Ind J Pharmacol 20: 221-222.
- Sultana N, Islam MT, Marcus de Alencar VOB, Silva SWC, Chowdhury MU, et al. (2015) Phyto-pharmacological screenings of two Rubiaceae family plants. Afr J Pharm Pharmacol 9(31): 775-782.
- Chea A, Jonville MC, Bun SS, Laget M, Elias R, et al. (2007) In vitro antimicrobial activity of plants used in Cambodian traditional medicine. Am J Chin Med 35: 867-873.
- Khairunnisa K, Karthik D (2014) Evaluation of in-vitro apoptosis induction, cytotoxic activity of Hymenodictyon excelsum (Roxb) Wall in Dalton's lymphoma ascites (DLA) and Lung fibroblast - Mouse L929 cell lines. J Appl Pharmaceut Sci 4(08): 11-17.
- Mishra Garima, Anjum Nishat, Tripathi YC, Pandey AK (2016) Antifungal and free radical scavenging efficacy of Hymenodictyon excelsum bark. Univerities' Journal of Phytochemistry and Ayurvedic Heights 1(20): 14-19.
- Kar B, Nepal A, Kumar RBS, Dolai N, Bhattacharya S, et al. (2013) Antioxidant and anti-inflammatory properties Hymenodictyon excelsum bark. Oriental Pharmacy and Experimental Medicine 13: 103-111.
- Chakraborty P, Tripathi YC, Tewari Devesh and Upadhyay L (2016) Phytochemical Evaluation and anti-inflammatory effects of Hymenodictyon excelsum (Roxb.) Wall grown in Uttarakhand State of India. Journal of Pharmacy Research 10(5): 199-204.
- Nepal A, Chakraborty M, Karmakar I, Bala A, Haldar PK (2016) Cytotoxic and anti proliferative activity of Hymenodictyon excelsum in ehrlich ascites carcinoma bearing mice: in vitro and in vivo studies. International Journal of Chemical and Pharmaceutical Analysis 3(2): 1-7.
- Biswakanth K, Abimanyu N, Suresh Kumar RB, Narayan D, Sanijb B, et al. (2013) Antioxidant and anti-inflammatory properties Hymenodictyon excelsum bark. Orient Pharm Exp Med 13: 103-111.
- Suruse PB, Duragkar NJ, Deshpande SA (2015) Evaluation of in vitro and in vivo antimalarial activity of Hymenodictyon excelsum bark extracts. Innovations in Pharmaceuticals and Pharmacotherapy (IPP) 3 (1): 554-560.
- Ishihara M, Yokote Y, Sakagami H (2006) Quantitative structurecy to toxicity relationship analysis of coumarin and its derivatives by semi empirical molecular orbital method. Anticancer Res 26: 2883-2886
- Rahman MM (2015) Evaluation of Hymenodictyon excelsum phytochemical's therapeutic value against prostate cancer by molecular docking study. Jundishapur J Nat Pharm Prod 10(1): e18216.






























