Nonconventional synthesis of fused Uracils:A review
Ajmal Rashid Bhat*
Department of Chemistry, S.B.B.S.University, India
Submission: April 23, 2017; Published: May 02, 2017
*Corresponding author: Ajmal Rashid Bhat, Department of Chemistry, S.B.B.S.University, Jalandhar Punjab-144030, India, Tel: +91-9797354802; Email: bhatajmal@gmail.com
How to cite this article: Ajmal R B.Nonconventional synthesis of fused Uracils: A review. Organic & Medicinal Chem IJ. 2017; 2(3): 555588.DOI:10.19080/OMCIJ.2017.02.555588
Abstract
Green chemistry has now become a subject of significant research in modern organic synthesis. The concept of "green chemistry" emerged in the early 1990s and is now widely used to meet the fundamental scientific challenges to protect the human health and environment while simultaneously achieving commercial feasibility. Nonclassical methods following the principles of green chemistry reduce or even eliminate the generation of hazardous substances and increasing product yield. Microwave (MW) and ultrasonic assisted techniques are the potential nonconventional techniques (green chemistry techniques) used during the recent years. These techniques are extensively used in organic chemistry, offering a versatile and facile pathway for a large variety of heterocyclic synthesis. This review paper highlights the synthesis of annulated uracils using microwave (MW) and ultrasound techniques. Compared with traditional conventional methods, this method is more convenient with high yields, short reaction times, mild conditions and easily controlled
Keywords: Microwave irradiation; Ultrasound irradiation; Annulated uracils; Benzaldehyde Derivativies; Cavitation
Introduction
I. Microwave assisted fused Uracils
The ability of MW-assisted organic synthesis to rapidly synthesize organic compounds is of significant benefit for library generation. Moreover, it allows modifications in selectivity (chemo-, regio-, and stereo-selectivity) and solvent, catalystfree conditions [1]. The synthesis of heterocyclic compounds have major role in medicinal chemistry research, from the past few decades; many significant advances in practical aspects of organic chemistry have included novel synthetic strategies and methods as well as advent of a vast array of analytical techniques. Hence, the present day chemists are no longer confined to using only thermal energy for driving chemical reactions. With increasing complexity of the problems and the availability of newer methods of activation of chemical reactions, chemist have restored to using microwave technique for rapid and efficient synthesis of a variety of compounds, because of selective absorption of microwave energy by polar molecules. The application of Microwave irradiation to provide enhanced reaction rate and improved product field in chemical synthesis and further showed the formation of a variety of carbonheteroatom bonds.
Microwaves couple directly with the molecules of the entire reaction mixture, leading to a rapid rise in the temperature. Since the process is not limited by the thermal conductivity of the vessel, the result is an instantaneous localized superheating of any substance that will respond to either dipole rotation or ionic conductivity. Only the reaction vessel contents are heated and not the vessel itself; better homogeneity and selective heating of polar molecules might be achieved. Following are the literature reported work of microwave assisted synthesis of N and O-type heterocyclic compounds.
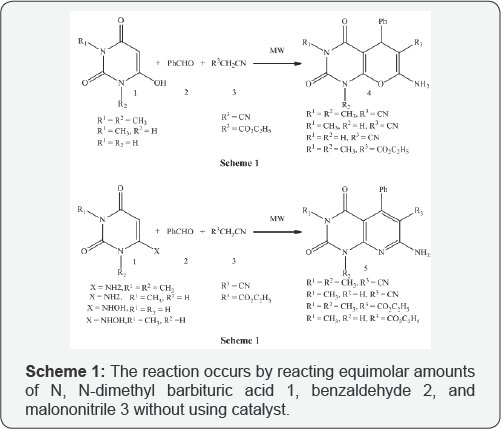
Ipsita et al. [2] reported a novel three component one pot synthesis of pyrano [2,3d] pyrimidines 4 and pyrido [2,3d] pyrimidines 5 under microwave irradiation at 60% power and 800C for 4-5 mins in the solid state. The reaction occurs by reacting equimolar amounts of N, N-dimethyl barbituric acid 1,benzaldehyde 2, and malononitrile 3 without using catalyst (Scheme 1).
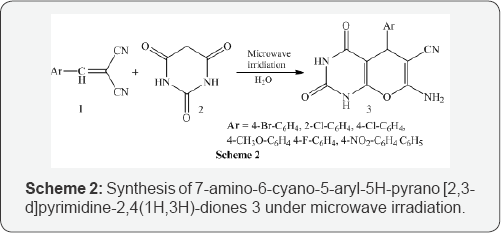
Yuan et al. [3] resulted effectives synthesis of 7-amino-6- cyano-5-aryl-5H-pyrano [2,3-d]pyrimidine-2,4(1H,3H)-diones 3 under microwave irradiation. A dry flask (25 mL) was charged with the arylidenemalononitrile 1, barbituric acid 2 and water (3 mL). The flask was then connected with refluxing equipment under microwave irradiation at power 285 W for 3-5 min (Scheme 2).
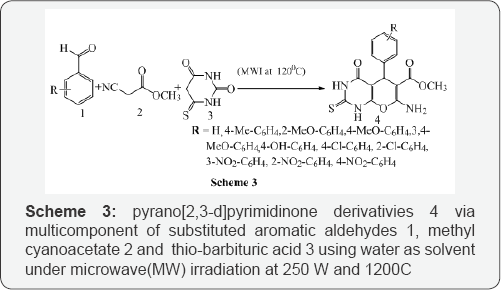
Bhat et al. [4] reported pyrano 2,3-d pyrimidinone derivativies 4 via multicomponent of substituted aromatic aldehydes 1, methyl cyanoacetate 2 and thio-barbituric acid 3 using water as solvent under microwave(MW) irradiation at 250 W and 1200C (Scheme 3). Shahrzad et al. [5] reported an efficient synthesis of Pyrido [2,3-d] pyrimidine derivatives 4 via one-pot three-component reaction of aromatic aldehyde 1,malononitrile 2, and 4(6)-aminouracil 3 under microwave irradiation in aqueous media (Scheme 4).
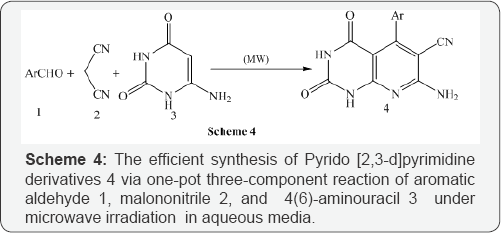
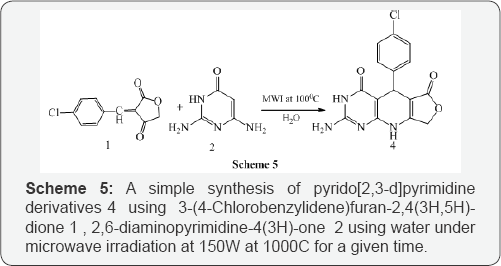
Jiang et al. [6] also reported a simple synthesis of pyrido 2,3-d pyrimidine derivatives 4 using 3-(4-Chlorobenzylidene) furan-2,4(3H,5H)-dione 1,2,6-diaminopyrimidine-4(3H)-one 2 using water under microwave irradiation at 150W at 1000C for a given time (Scheme 5). Mont et al. [7A & 7B] synthesized functionalized pyrido [2,3-d]Pyrimidines 4 via cyclocondensation α,β-unsaturatedesters 1, active methylene compounds 2 (malononitrile, or methyl cyanoacetate) and guanidinium carbonate 3 under microwave-irradiation at 1400C (Scheme 6).

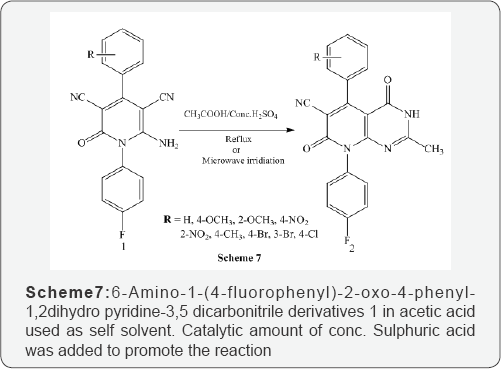
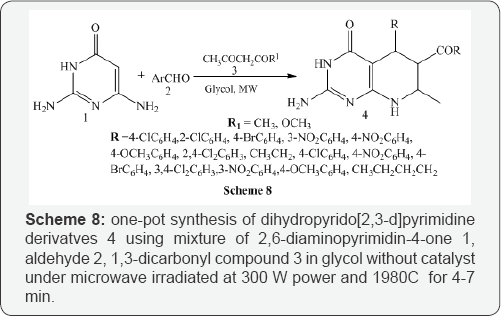
Kamlesh et al. [8] synthesized microwave and conventional novel pyrido[2,3-d]pyrimidine derivatives 2 using 6-Amino-1-(4-fluorophenyl)-2-oxo-4-phenyl-1,2dihydro pyridine-3,5 dicarbonitrile derivatives 1 in acetic acid used as self solvent. Catalytic amount of conc. Sulphuric acid was added to promote the reaction. The reaction mixture was heated on oil bath at reflux temperature and the same reaction mixture was also monitored under microwave irradiated at 180 MW (Scheme 7). Shujiang et al. [9] also reported microwave-assisted one- pot synthesis of dihydropyrido[2,3-d]pyrimidine derivatves 4 using mixture of 2,6-diaminopyrimidin-4-one 1, aldehyde 2,1,3-dicarbonyl compound 3 in glycol without catalyst under microwave irradiated at 300 W power and 1980C for 4-7 min (Scheme 8).
Kamlesh et al. [10] reported the microwave assisted synthesis of novel pyrido[2,3-d]pyrimidines using dihydropyridines. The reaction mixture was at180 W power (Scheme 9).


Shujiang et al. [11] synthesized an efficient route of new class of pyrido[2,3-d]pyrimidine derivatives 3 using reaction mixture of 4-arylidene-3-methylisoxazol-5(4H)- one 1, 2,6-diamino- pyrimidin-4(3H)-one 2, DMF and HOAc at 240 W power and 140OC temperature (Scheme 10).
Galve et al. [12] reported the synthesis of 2-arylamino substituted4-amino-5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)- ones 6 from treatment of pyridones 3 (synthesized from α, β-unsaturated esters 1 and malononitrile 2) with the aryl guanidines 4 to form 3-aryl substituted pyridopyrimidines 5, which undergoes Dimroth rearrangement by NaOMe/MeOH. The overall yields of such α β-step protocol are higher than those of the multicomponent reaction between α, β-unsaturated ester 1, malononitrile 2, and an aryl guanidine 4 (Scheme 11).

II. Ultrasonic assisted fused Uracils
The study of ultrasound is concerned with understanding the effect of sound waves and wave properties on chemical systems. Ultrasound ranges from about 20 to 10 MHz and can be roughly subdivided in three main regions: low frequency high power ultrasound (20-100 kHz), high frequency medium power ultrasound (100 kHz-1 MHz), and high frequency low power ultrasound (1- 10 MHz). It is transmitted through any substance such as, solid, liquid or gas, which possesses elastic properties. The movement of the sound source is transmitted to the particles of the medium, which oscillate in the direction of the wave and produce longitudinal waves as well as transverse waves.
As the molecules of the medium vibrate, the average distance between the molecules decreases in the compression cycle and increases during rarefaction. When the average distance between the molecules exceeds the critical molecular distance necessary to hold the liquid intact, the liquid breaks down; cavities (cavitation)' and bubbles are formed. This process, known as cavitation, refers to the formation and the subsequent dynamic life of bubbles in liquids. Therefore cavitation is the formation, growth, and implosive collapse of bubbles irradiated with sound, which is the impetus for sonochemistry. The bubbles can be filled with gas or vapour and occur in water, organic solvents, biological fluids, liquid helium, molten metal's or other fluids. Bubble collapse results in high temperature (as much as 47000C) and pressure changes (10 Pa). The solvent/reagent vapour suffers fragmentation to generate reactive species, such as free radicals or carbenes. These high-energy species are concentrated at the interface and lead to intermolecular reactions. However, if there are in volatile solutes, they would also collect at the interface and react with the high energy species. Besides this, the shock wave produced by a bubble collapse can influence the reactivity by altering the solvation of the reactive species.
A number of common reactions used in synthetic heterocyclic chemistry have carried out more efficiently using ultrasound techniques. There are several advantages. Generally, the yield increases and the percentage of by-products decrease [13a & 13b]. Reactions occur faster, so that lower temperatures can be used.
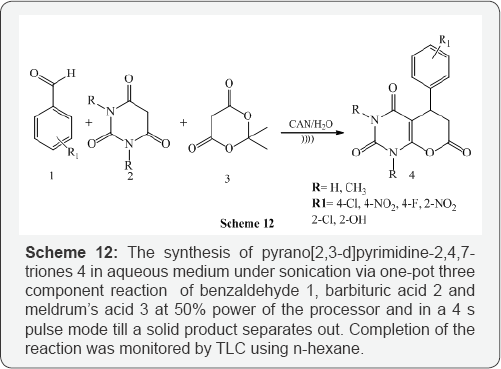
Anshu et al. [14] used Cerric ammonium nitrate (CAN) as efficient catalyst for the synthesis of pyrano[2,3-d]pyrimidine- 2,4,7-triones 4 in aqueous medium under sonication via one-pot three component reaction of benzaldehyde 1, barbituric acid 2 and meldrum's acid 3 at 50% power of the processor and in a 4 s pulse mode till a solid product separates out. Completion of the reaction was monitored by TLC using n-hexane: ethyl acetate (7:3) as an eluant. All the reactions were invariably completed in 25-40 min. (Scheme 12).
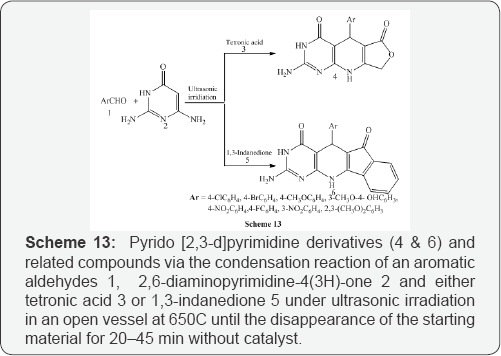
Shujiang et al. [15] reported a series of pyrido[2,3-d] pyrimidine derivatives (4 & 6) and related compounds via the condensation reaction of an aromatic aldehydes 1,2,6- diaminopyrimidine-4(3H)-one 2 and either tetronic acid 3 or 1,3-indanedione 5 under ultrasonic irradiation in an open vessel at 650C until the disappearance of the starting material for 20-45 min without catalyst. This protocol has the advantages of higher yields, lower cost and convenient procedure (Scheme 13).
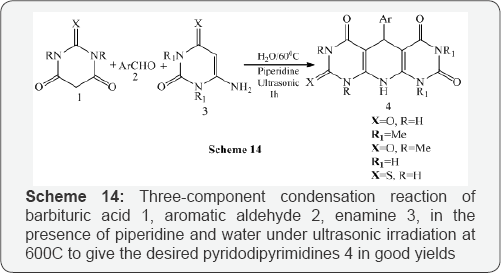
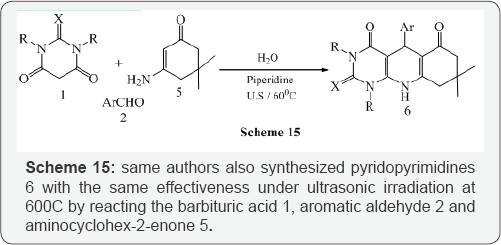
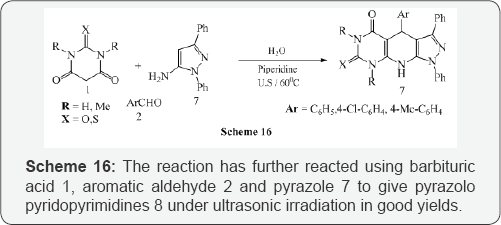
Mosslemin et al. [16] reported a one-pot, three-component condensation reaction of barbituric acid 1, aromatic aldehyde2, enamine 3, in the presence of piperidine and water under ultrasonic irradiation at 600C to give the desired pyridodipyrimidines 4 in good yields (Scheme 14).The same authors also synthesized pyridopyrimidines 6 with the same effectiveness under ultrasonic irradiation at 600C by reacting the barbituric acid 1, aromatic aldehyde 2 and aminocyclohex-2-enone 5 (Scheme 15). The reaction has further reacted using barbituric acid 1, aromatic aldehyde 2 and pyrazole 7 to give pyrazolo pyridopyrimidines 8 under ultrasonic irradiation in good yields (Scheme 16).
Conclusion
Non conventional techniques (Ultrasound and microwave irradiation) provide alternative pathways for reactions, due to the formation of high energy intermediates. Ultrasound is, thus, an alternative method for rapid and facile synthesis of a variety of heterocycles and fused heterocycles. Ultrasonic-assisted organic synthesis (UAOS) is a powerful and green approach which is being used more and more to accelerate synthesis of organic compounds. However, it has significant applications in various fields such as chemical synthesis, pharmaceutical industry, food and polymer industry, electroplating, decontamination etc. Compared with traditional conventional methods, this method is more convenient with high yields, short reaction times, mild conditions and easily controlled. Synthetic applications describes a variety of new chemistries that can be performed with microwave irradiation, but a wide range of microwave- assisted applications is still waiting. There have been tremendous successes in the synthesis of numerous numbers of heterocyclic compounds and in the development of new processes under clean, environmentally benign methodologies that are sustainable for the long term.
References
- De-Hoz A, Diaz-Ortiz A, Moreno A, S-Migallon P, Prieto J R, et al. (2007) Microwave-assisted reactions in heterocyclic compounds with applications in medicinal and supramolecular chemistry. Comb Chem High Throughput Screening 10: 877-902.
- D Ipsita, B S D Kumar, J B Pulak (2003) A novel three-component one-pot synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d] pyrimidines using microwave heating in the solid state. Tetrahedron Lett 44: 8307-8310.
- G Yuan, T Shujang, L Tuanjie, Z Xiaojing, Z Songlei, et al. (2004) Daqing, Effective Synthesis of 7-Amino-6-cyano-5-aryl-5H-pyrano[2,3-d] pyrimidine- 2,4(1H,3H)-diones Under Microwave Irradiation. Synth Commun 34: 1295-1299.
- A R Bhat, A H Shalla, R S Dongre (2015) Microwave assisted one-pot catalyst free green synthesis of newmethyl-7-amino-4-oxo-5-phenyl-2- thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carboxylates as potent in vitro antibacterial and antifungal activity. J Adv Res 6: 941948.
- A Shahrzad, B Saeed, Intern (2012) An Efficient Synthesis of Pyrido[2,3-d]pyrimidine Derivatives via One-Pot Three-Component Reaction in Aqueous Media. J Org Chem 2: 7-14.
- T Shu-Jiang, Y Zhang, H Jiang, B Jiang, Z Jun-Yong, et al. (2007) A Simple Synthesis of Furo[3,4:5,6]pyrido[2,3-d]pyrimidine Derivatives through Multicomponent Reactions in Water. Eur J Org Chem 1522-1528.
- A. N Mont, J Teixid, J I Borrell, C O Kappe (2003) A three-component synthesis of pyrido[2,3-d]pyrimidines. Tetrahedron Lett 44: 53855387.
- B. N Mont J Teixid C O. Kappe, J I Borrell (2003) A one-pot microwave assisted synthesis of pyrido[2,3-d]pyrimidines. Mol Divers 7: 153-159.
- M K Kamlesh, M G Ravindra, T K Taslimahemad, K P Praful (2014) Microwave and conventional synthesis of novel pyrido[2,3-d] pyrimidine scaffold as an antimicrobial agent. Chem & Bio Interface 4: 119-130.
- T Shujiang, Z Junyong, X Zou, F Fang, L Tuanjie (2005) Microwave- assisted one-pot synthesis of 2-amino-6,7-disubstituted -5-methyl-5,8- dihydropyrido[2,3-d]pyrimidin-4(3H)-one without catalyst. Arkivoc 76-81.
- K Kamlesh, K Taslim, R Vijay, P Praful (2013) Microwave assisted synthesis and biological investigations of novel derivatives of pyrido[2,3-d]pyrimidines. Chemistry & Biology Interface 3: 192-200.
- T Shujiang, Z Junyong, J Runhong B Jiang, Y Z Hong (2007) An efficient route for the synthesis of a new class of pyrido[2,3-d]pyrimidine derivatives. J Org Biomol Chem 5: 145-1453.
- I Galve, R P D-Bellacasa, D Anchez-Garc, X Batllori, JT Borrell (2012) Synthesis of 2-arylamino substituted 5,6-dihydropyrido[2,3-d] pyrimidine-7(8H)-ones from arylguanidines. J Mol Divers 16: 639-649.
- a. H M Mohammad, R N Mohammad (2010) Rapid and efficient synthesis of fused heterocyclic pyrimidines under ultrasonic irradiation. Ultrason Soochem 17: 162-167.
- b. BK Jadidi, R Gharemanzadeh, M Mehrdad, HR Darabi, H R Khavasi, et al. (2008) A facile synthesis of novel pyrrolizidinee under classical and ultrasonic conditions. Ultrason Sonochem 15: 124-128.
- D Anshu, L Shyam, S B Gupta (2013) Cerric ammonium nitrate (CAN) catalysed synthesis of pyrano[2,3-d]pyrimidine-2,4,7-Triones in aqueous medium under sonication via one-pot three component reaction. Eur Chem Bull 2: 836-841.
- Shujiang, C Longji, Z Yan, S Qingqing, Z Dianxiang, et al. (2008) An efficient synthesis of pyrido[2,3-d]pyrimidine derivatives and related compounds under ultrasound irradiation without catalyst. Ultrason Sonochem 15: 217-221.
- M H Mosslemin, M.R Nateghi (2010) Rapid and efficient synthesis of fused heterocyclic pyrimidines under ultrasonic irradiation. Ultra Sonochem 17: 162-167.






























