Topical Salicylic acid and Lactic acid Microemulsion
Maher Aljamal12*, Ibrahim Kayali2 and Mohammad Abul-Haj2
1BeitJala Pharmaceutical Company /Research and Development department, Palestine
2Department of Chemistry, Palestine, Al- Quds university, Israel
Submission: April 26, 2017; Published: May 02, 2017
*Corresponding author: Maher Aljamal, BeitJala Pharmaceutical Company / Research and Development department, Al- Quds university, Palestine, Tel: 00972- 0598079095; Email: maheraljamal2013@yahoo.com
How to cite this article: Maher A*, Ibrahim K, Mohammad A-H.Topical Salicylic acid and Lactic acid Microemulsion . Organic & Medicinal Chem I J. 2017; 2(3): 555587. DOI: 10.19080/OMCIJ.2017.02.555587
Abstract
Microemulsionsare used to solubilizing and improve an active pharmaceutical ingredientssolubility and permeability, such as those for topical absorption availability. The objective of this study was to prepare a microemulsion liquid composed of 12% salicylic acid and 4% of lactic acid using castor oil, Tween 80, propylene glycol, ethyl alcohol and purified water. Using the low energy emulsification technique; four pseudo ternary phase diagrams were constructed and studied for at least 75 days under a titration method using purified water (with or without propylene glycol), each phase diagram was investigated at 25°C, 37°C and 45°C. The phases include conventional emulsion, viscous and transparent/translucent microemulsion. The Results indicate a clear, thermodynamic microemulsion liquid obtained in each of the constructed phase diagrams at all temperatures of study. Using propylene glycol as a co-surfactant, lead to more stable microemulsionsby using low concentration of Tween 80. It was suggested that microemulsions of 12% Salicylic acid and 4% Lactic acid could be a suitable vehicle for topical treatment of psoriasis, scaly patches, ichthyoses, dandruff, corns, calluses, and wartson the hands or feet. This study may be useful in formulating a delivery system for the pharmaceutical industry as well as in cosmetics and personal care products.
Keywords: Pseudo ternary Phase diagram; Microemulsion; Salicylic acid; Lactic acid; Skin conditions; Scaly skin
Introduction
Microemulsions are clear, thermodynamically stable, optically isotropic systems consisting of at least one hydrophilic, one hydrophobic and an amphiphilic component (surfactants/ co-surfactants)[1]. Microemulsions were used in several fields as routes of administration to deliver a drug in a sustained or controlled manner for prolonged delivery, as compared to conventional dosage form for topical applications which exhibit minimal systemic absorption. Microemulsion can be prepared using a low-energy emulsification method, depending on the phase behavior and the properties of the constituents, to promote the formation of highly small droplets using cosurfactants that include self-emulsification. The low energy technique can be introduced using the phase behavior and studying its available applications for the topical treatment of warts, viruses, corns and calluses. The proposed microemulsion drug will include an improvement in poorly drug solubility, enhancement of bioavailability, protection of the unstable drugs against environmental conditions and a long shelf life can be obtained. [2].
It was found that a topical preparation composed of 12% Salicylic acid and 4% lactic acid in colloidional base may be beneficial in speeding resolution of a viral infection [3].Antibacterial activity of Salicylic acid was recorded by Park et al. [4] and Kupferwasser et al. [5] as adjuvant therapeutic agent in treatment of viral infections. Salicylic acid is used topically in hyperkeratotic and scaling conditions to enhance the rate of loss of surface scales [6]. Salicylic acid is readily absorbed through the skin, slowly excreted in urine; and systemic toxicity resulting from application to large area of the skin has been reported [7]. The side effects of salicylic acid include sensitivity, excessive drying, and irritation. Traditional formulations of salicylic acid in ointment bases have disadvantages of being greasy and irritant due to free crystals of the drug.
Salicylic acid is the main therapeutic catalytic compound in the proposed microemulsion drug which is used for a number of different skin conditions caused by thickened, hard skin, such as warts, verrucas, psoriasis, scaly skin conditions and some nail infections. In which it works by softening the outer layer of skin allowing it to loosen and shed [8]. While Lactic acid used in severe forms of dry scaly skin since it is a humectants and supporter (booster catalytic) in the medication product [9].
There are three conventional effects of an alcohol additive in the formulation of microemulsion that have been mentioned so far [10]. First, it contributes to the general formulation as a co-surfactant, slightly hydrophilic contribution for methanol and ethanol; lipophilic contribution for n-butanol and longer linear alcohols, Secondly, as a co-solvent. The alcohol will be adsorbed with the surfactant at the interface and changes the overall interaction of the amphiphilic film with the adjacent solvents. When such an alcohol co-solvents are present in small proportion, they might not mix uniformly in the bulk of the oil or water phase and they could exhibit a third effect calledlipophilic linker. Alcohols in microemuslion formulations has been employed as co-surfactants and as co-solvent in order to decrease surfactant film rigidity and to reduce the time needed for equilibration to be reached in multi-phase systems [11]. Microemulsionstabilized by sugar surfactants usually contains a co-surfactant. Since without a co-surfactant only o/w microemulsions are formed, which is mainly due to the fact that the high hydrophilicity of sugar surfactants cannot be changed significantly by temperature variation [12].
In this study, 12% salicylic acid was incorporated with 4% lactic acid using Castor oil, Tween 80, propylene glycol, ethyl alcohol and water. The characterization of the prepared systems in each phase diagram was carried out by applying visual inspection evaluation tests to examine the appearance of microemuslion.
Instrumentation & Methodology
I. Instruments
Analytical balance (Precisa), Vortex (VELP), culture tubes sealed with Vito lined screw caps, Incubators (WTB Binder), crossed polarizer's and Dynamic light scattering.
II. Materials
Salicylic acid100% (Rhodia), Lactic acid90% (Merck), Ethanol 96% (shitzer), polysorbate 80or Tween 80 (Eigenmann&Veronelli), purified water, castor oil (gustavheess) and propylene glycol (Dow).
III. Phase diagram Methodology
A. Salicylic acid is slightly soluble in water and soluble in ethanol, while lactic acid is soluble in water and in ethanol. Therefore the microemulsion active pharmaceutical ingredients target concentration (12% salicylic acid and 4% lactic acid) were dissolved in 50 ml of ethanol, to satisfy the solubility for both ingredients in a solution named Ethanolic solution.
i. Constructing of Phase Diagrams: The pseudo ternary phase diagrams consisting of Tween 80, Ethanolic solution (with or without castor oil) and purified water (with or without propylene glycol) were constructed using the titration method, each of them representing an apex of the triangle [13]. Ternary mixtures with different weight ratios of Tween 80 and Ethanolic solution (with or without castor oil) were prepared, each mixture in a separate culture tube sealed with viton lined screw. These samples mixtures were titrated individually with purified water (with or without propylene glycol)which was added drop by drop in increments of (4%, 8%, 12%, 16%, 20%, 24%, 28%, 32%, 36%,40%, 44%, 48%, 52%, 56%, 60%, 64%, 68%, 72%, 76%, 80%, 84%, 88%, 92%, 96%) using calibrated presica balance at room temperature (25± 2°C) until solubilization limit was reached. Vigorous stirring followed after each addition using a vortex mixer. The equilibration time between each addition was taken typically after 24 hours and before the next titrant addition of purified water (with or without propylene glycol). The readings were determined using visual inspection and cross polarizers. Each ternary mixture prepared was investigated at three temperatures 25°C, 37°Cand 45°C. To detect the boundary of single phase; four phase diagrams were installed and each phase was tested at three temperatures 25°C, 37°C and 45°C. The clear mixtureswere considered desirable and having smallest droplet size.
ii. Visual inspection: Visual inspection was made after each addition. The samples were identified as microemulsions when they appear as transparent/translucent and easily flow able liquid. The samples were identified as emulsions when they appeared as milky or turbid liquids. All these categories were plotted on atri angular graph as ternary or pseudo ternary phase diagram using origin pro 8 computer programs.
Results and Discussions
Microemulsion phase diagram results
A wide variety of structures and phases can be formed by mixing oil, water and surfactants in differentratios. Molecular and structural examinations, concentration of surfactants and other ingredients can expose the existence of microemulsions structure depending on the ratio of the components. The acknowledgement of different phases and structures can be achieved by simple visual inspection of their physical appearance (e.g., microemulsions are transparent/translucent, emulsions are non transparent and phases separate after a while) [14,15].
Nonionic surfactant such as Tween 80 in particular is safe agent for all biological tissues in general and for skin specifically [16,17], for topical drug applications. This nonionic surfactant is compatible with various ingredients used in the preparation of microemulsions and is not affected by pH. The increase in the surfactant concentration, will lead to an increase in the observed emulsifying region. Tween 80 of 15 HLB value was selected as a main surfactant and propylene glycol of 2.5 HLB value was selected as a co-surfactant that matches castor oil and provides to lowest the interface tension between castor oil and water phases.
Propylene glycol and short-chain alcohols (as ethyl alcohol) are known to act as co-surfactants. Hence, it is supposed that a considerable part of propylene glycol is incorporated into the surfactant layer and will increase the interfacial fluidity, and the other part of propylene glycol will decrease the polarity of the water phase because propylene glycol is mainly soluble in water. Propylene glycol is hydrophilic, hense soluble in water but practically non-soluble in the oil phase [18]. In comparison with other alcohols, propylene glycol is relatively tolerable by the skin. Propylene glycol as a co-surfactant helps Tween 80 to form more stable microemulsion since propylene glycol has an affinity for castor oil and water phases and partitions to an appreciable extent into the surfactant. Propylene glycol will contribute to the low interfacial tension monolayers at the oil/water interface which is required for formation of microemulsion.
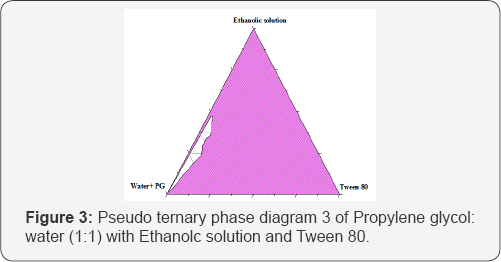
Different combinations of castor oil, tween 80, propylene glycol, ethyl alcohol and water when mixed together produce typical microemulsion in the form of o/w or w/o. Pseudoternary phase diagram construction is the best way to study all types of formulations that can originate from mixing of all above ingredients. This study is performed to predict the optimized compositions of the ingredients in the development of microemulsion. (Figures 1-4) are the determined pseudoternary phase diagrams mixtures. A large area of transparent/clear microemulsion solution was formed in the oil rich regions. The microemulsion areas were found in all figures to be close to the boundaries of the diagrams where water and castor oil ratios were low and the surfactant ratio was high. By increasing tween 80 or propylene glycol concentration, the microemulsionregion becomes larger. This can be explained on the basis of the enhancement in micelle formation with the increase in surfactants ratio, which consequently increases the solubilizing capacity of the microemulsion.
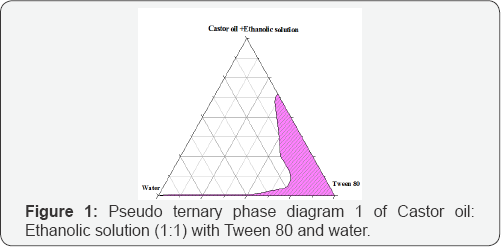

In Figure 3&4 the incorporation of propylene glycol as a co-surfactant increased the maximum amount of combined water in castor oil+ Ethanolic solution-tween 80 system with them icroemulsion zone being increased compared to the surfactant system as shown in Figures 1&2. Water in oil (W/O) microemulsion occupied the lower right region of the triangle phase diagram (Tween 80 rich region). The formation of W/O microemulsion or O/W microemulsion depends on the composition of Tween 80 and its solubility in castor oil and water. The results indicated that a mixture of two non-ionic surfactants with large difference in HLB values between them might be able to produce more stable microemulsion preparations. This may be due to the fact that surfactants with very low HLB value(propylene glycol) dissolved in oil phase and the surfactant with high HLB value (Tween 80) dissolved in the water phase that enable them to function together well enough to extend stronger effect than surfactant and co-surfactant mixture having closer HLB values ) [7].
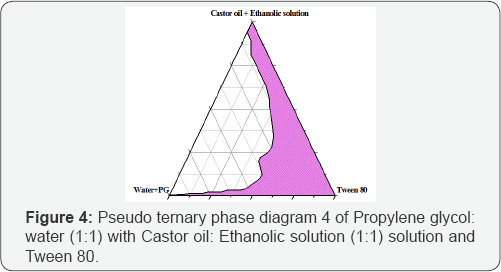
It can be observed in Figures 1 that the water content inthe system was in the range of 4 to 25%. It was found that water content above 25% was insufficient to hydrate the polyoxyethylene groups, which iscritical for the swelling of surfactant chains to demonstrate the microemulsion area. When the water and propylene glycol content was above 50% as in figure 4, the distance between the polyoxyethylenegroups decreased and destabilized the microemulsion are sulting in breaking of the swelled microemulsion. With increasing water content above 60% at lower concentrations of surfactant, the samples begin to lose their transparency becoming more and more turbid and there is separation among the two phases.
The microemulsion region was found clear and transparent liquid in all prepared formulations and at all temperatures of study, this is because in the initial stage of titration with water (with or without propylene glycol) for each mixture tube at the ratio of 0.5:9.5, 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1, 9.5:0.5 (Wt/Wt) shows a microemulsion region by means of low concentration of castor oil. When water (with or without propylene glycol) was added drop wise, the castor oil and water (with or without propylene glycol) disperse in each other properly and no phase separation of such molecules due to high concentration of surfactant occurs and therefore clear and transparent microemulsion was formed. The higher the HLB surfactant number is the easier to dissolve in water in which it has the ability to form O/W microemulsion. The phase behavior of microemulsion region for each phase diagram formula was investigated at 25°C, 37°C and 45°C and the microemulsion region remains the same. Therefore, an increase in the temperature will not have an effect on the non-ionic surfactant micelle structure (W/O).
Conclusion
Incorporation of salicylic acid with lactic acid into microemulsion liquid was obtained in the four pseudoternary phase diagrams formulations. By increasing the surfactant concentration, the microemulsion region becomes larger. By using propylene glycol as a co-surfactant, the microemulsion region becomes larger in the initial stage of titration for each mixture ratio. The results indicated that a mixture of two non-ionic surfactants with large difference in HLB values between them might be able to produce much more stable, isotropic and larger microemulsionregion. The microemulsion retained its characters at ambient temperature conditions and at intermediate and higher temperatures, 37°C and 45°C, respectively. The results suggested that microemulsion could be a suitable vehicle for topical application of 12% Salycilic acid and 4% Lactic acid. This study will be very useful in formulating a deliverysystem in pharmaceutical industry as well as incosmetics and personal care products.
Research Problem
Microemulsions were used in several fields as routs of administration; this research application, which delivered the drug in sustained or controlled manner and prolonged delivery as compared to conventional dosage form for topical applications to exhibit minimal systemic absorption. Microemulsion can be prepared using a low-energy emulsification method, depending on the phase behavior and properties of the constituents, to promote the formation of high small droplets using co-surfactants that include self emulsification. So, why not using? the low energy technique in our medicine factories?, and introduce the new nanotechnology in Palestinian medicine factories as seen in all over the world in general using the phase behavior and studying its available applications for the topical treatment of warts, verrucas, corns and calluses.
Acknowledgement
I would like to thank BeitJala Pharmaceutical Company, where I work, represented by the general manager Dr. Angele Zaboura, who is gently pushing me to pursue something greater
References
- Danielsson B, Lindman (1981) "The definition of a microemulsion, Colloids and Surfaces” 391-392.
- JorgKreuter (1994) "Colloidal Drug Delivery Systems”. 66: 31-71.
- Leslie KS, Dootson G, Sterling JC (2005) "Topical salicylic acid gel as a treatment for molluscumcontagiosum in Children”. J Dermatolog Treat 16: 336-40.
- Park WB, Kim SH, Cho JH, Bang JH, Kim HB, et al. (2007) "Effect of salicylic acid on invasion of human vascular endothelial cells by Staphylococcus aureus. FEMS Immunol Med Microbiol” 49: 56-61.
- Kupferwasser LI, Yeaman MR, Nast CC, Kupferwasser D, Xiong YQ, et al. (2003) "Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathway in Staphylococcus aureus”. J Clin Invest 112(2): 222-233.
- British Medical Association and the Royal Pharmaceutical Society of Great Britain (1993) "British National Formulary” 26: 408-410.
- Reynolds JEF, editor. Martindale, the extra pharmacopoeia (1993) "London: The Pharmaceutical Press” 30th edition.
- Helen Allen (2015) "Salicylic acid” 3739(v25)
- Michael Boivin BSc Phm (2009) "Managing dry skin-Teva Pharmacy Solutions” 1.0 CEU.
- CosimaStubenrauch (2009) "Microemulsions Background, New Concepts, Applications, Perspectives”, 1 st Ed. A John Wiley and Sons Ltd, Inc., New Delhi, India.
- Ibrahim Kayali (2012) "Water-Diesel Microemulsions Stabilized by an Anionic Extended Surfactant and a Cationic Hydrotrope ". Journal of Dispersion Science and Technology 33: 516-520.
- Cristobal Carnero Ruiz (2009) "Suger-Based Surfactant: Fundamentals and Applications” 1st Ed. Taylor & Francis Group. London. UK.
- Osborne DW, Middleton CA, Rogers RL, J Disper Sci Technol (1988) "Identification of phases of various oil, surfactant/co-surfactants and water system by ternary phase diagram). Acta Pol Pharm 9: 415.
- Badawi AA, Nour SA, Sakran WS, El-Mancy SS (2006) "Formulation and evaluation of new emulsion systems as dosage forms”, MD thesis submitted to faculty of pharmacy, Cairo University. Egypt.
- Malcolmson C, Lawrence M (1995) "Three-component nonionic oilin water microemulsions using polyoxyethylene ether surfactants”,Colloids Surf., B Biointerfaces 4: 97-109.
- Constantinides PP, Scalart JP, Lancaster C, Marcello J, Marks G, Ellens H, et al. (1994) "Formulation and intestinal absorption enhancement evaluation of water-in-oilmicro emulsionsin corporating medium- chain glycerides”11(10):1385-1390.
- Constantinides PP, Welzel G, Ellens H, Smith PL, Sturgis S, YivSH, et al. (1996) "Water-in-oil microemulsions containing medium- chainfatty acids/salts: formulation and intestinal absorption enhancementevaluation”. Pharmaceutical Research 13(2): 210-215.
- Nour SA, Shalaby SH, Afify NN, Abd El-Aal S, Mekhael MK. (2002) "Formulation and evaluation of econazole nitrate emulgels”, J Drug Res Egypt 24(1-2): 63-71.






























