Design, Synthesis, and Biological Evaluation of Mutual Prodrugs of Some Selected NSAIDs
Nidhi Sharma1, Anil Kumar Sahdev2 and Vinit Raj2
1Dr. K N Modi Institute of Pharmacy & Research Centre, Modinagar, India
2Department of Pharmaceutical Sciences, Babasaheb Bhimrao Ambedkar University, India
Submission: March 26, 2017; Published: April 25, 2017
*Corresponding author: Vinit Raj, Department of Pharmaceutical Sciences, Babasaheb Bhimrao Ambedkar University, Vidya Vihar, Rae Bareli Road, Lucknow 226025, India, Tel 8859383897; Email: raj.vinit24@gmail.com
How to cite this article: Nidhi S, Anil K S, Vinit R. Design, Synthesis, and Biological Evaluation of Mutual Prodrugs of Some Selected NSAIDs. Organic & Medicinal Chem IJ. 2017; 2(3): 555586.DOI:10.19080/OMCIJ.2017.02.555586
Abstract
Non-steroidal Anti-inflammatory drugs (Aceclofenac, Mefenamic acid) have conjugated with different antioxidants such as Thymol, Menthol which having antiulcerogenic activity with the NSAIDs- antioxidant mutual prodrug gastro sparing NSAIDs devoid of Ulcerogenic side effects. Two mutual Prodrugs have synthesized in which NSAIDs have been conjugated with different antioxidants by a glycolic acid spacer. The structure was confirmed by using IR, NMR (1H-NMR) & Characterized by some physicochemical properties including the Melting point. Titled compounds showed the significant protection towards inflammation as well as exhibited potential antibacterial activity.
Keywords: NSAIDs; Mutual Prodrug; Ulcerogenic; Antioxidants
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most widely used medicines in the world, because of their analgesic, anti-inflammatory, and antipyretic activities [1,2]. However, the use of NSAIDs results in serious upper gastrointestinal (GI) adverse effects [3]. The pharmacological activity of NSAIDs is related to their ability to inhibit the activity of the enzyme cyclooxygenases (COXs) involved in the biosynthesis of prostaglandin mainly prostaglandin H2 (PGH2) [4]. It is well known that COX exists in two is forms, namely COX-I and COX- II, which are regulated differently [5]. COX-I is constitutively expressed in the stomach to provide cytoprotection in the GIT [6]. COX-II is inducible and plays a major role in prostaglandin biosynthesis in inflammatory cells [7]. Since most of the NSAIDs used clinically inhibit both is forms, long-term use of these agents results in gastric ulcer and there is enough evidence that inhibition of COX-I rather than that of COX-II underlies gastric ulcer formation [8].
But initial enthusiasm for selective COX-II inhibitors as safer NSAIDs has faded due to the emergence of serious cardiovascular side effects on long term use and need for design and development of safer agents still remain [9,10].
It has been well known that local generation of various reactive oxygen species (ROS) plays a significant role in the formation of gastric ulceration associated with NSAID therapy [11]. These observations indicate that antioxidants may be used to prevent NSAIDs-induced gastric ulcers. During the past few decades, a large number of naturally occurring compounds have been identified as antioxidants like Thymol, menthol, which are viewed as promising therapeutic agents for treating free radical mediated diseases including NSAID-induced peptic ulcers [12].
A Large number of herbs and spices are recognized as a source of natural antioxidants and studies have confirmed their efficacy for the treatment of gastrointestinal ulcers [13]. Based on these observations, it has been suggested that co administration of antioxidants and NSAIDS in formulated dosage form may possibly decrease the risk of NSAIDs induced gastrointestinal side effects [14].
However, there are potential advantages in giving such coadministered drugs having complementary pharmacological activities in the form of a single chemical entity. Such agents are named as mutual prodrugs which are designed with improved physicochemical properties [15,16]. In the view of this background, the present study was mutual prodrugs of NSAIDs with different antioxidants to get NSAIDs with lesser ulcerogenic side effects while retaining the anti-inflammatory and analgesic activity [17].
A mutual prodrug is a form of the drug in which two pharmacologically active agents are attached to each other in such a way that each drug acts as a moiety/carrier for each other and vice versa. The association may be “synergistic” if the carrier shows the same biological action as that of parent drug or may provide “additional” benefit if it shows new pharmacological action which is lacking in parent drug (Figures 1-3).
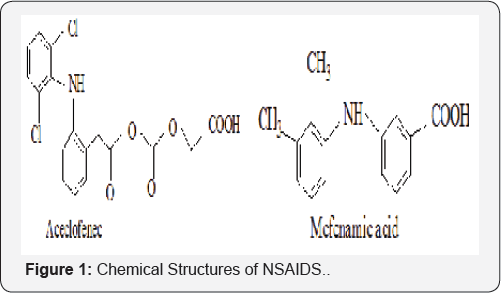
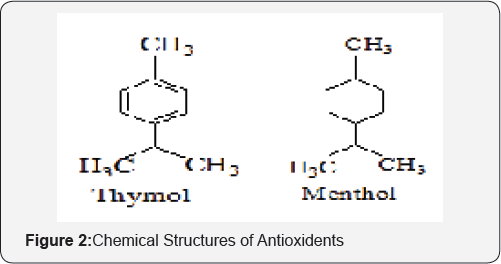

Experimental Procedure
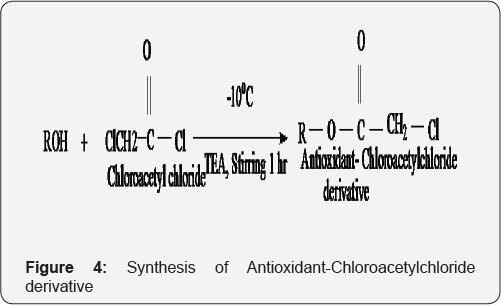
A. Synthesis of Antioxidant-Chloroacetylchloride derivative
A mixture of an antioxidants (like 1.563 gm/mol Menthol, 1.502 gm/molThymol) (0.01 mol) and TEA (1.0119 ml, 0.01 mole) in dichloromethane (25ml) was cooled in an ice-salt mixture to 10 °C. To this reaction mixture, chloroacetyl chloride (1.1294 ml, 0.01 mole) in Chloroform (25ml) was added drop wise with constant stirring over a period of 1 h, maintaining the temperature constant. The reaction mixture was stirred overnight at room temperature, washed with 5% HCl (3x50 ml), 5% NaOH (3x50 ml) and finally with brine solution (2x25ml). The organic layer was dried over anhydrous sodium sulfate, filtered and the solvent was removed under reduced pressure to obtain the corresponding antioxidant-chloroacetyl derivative. In this procedure, two derivatives of different antioxidants were prepared. These derivatives were recrystallized from petroleum ether and ethyl acetate (Figure 4).
B. Synthesis of NSAIDs-antioxidant mutual Prodrugs

A mixture of above reaction compound (0.01mole), NSAID (like Mefenamic acid, Aceclofenac) (0.01mole), TEA (0.01mole) and sodium iodide (0.01mole) in DMF (25 ml) was stirred overnight at room temperature. The reaction mixture was poured into finely crushing ice with stirring and extracted with chloroform (4x25ml). The combined organic layer was washed with 2% sodium thiosulphate (3x25ml), 5% HCl (3x50ml), 5% NaOH (3x50 ml) and finally with brine solution (2x25ml). The organic layer was dried over anhydrous sodium sulphate, filtered and the solvent was removed under reduced pressure to obtain the NSAIDs- antioxidant mutual prodrugs. The final producstwere obtained as solids and recrystallized from petroleum ether and ethyl acetate. The percentage yield, physical appearance, melting point and TLC are listed in the Table 1 (Figure 5).

C. IR data of the final compound
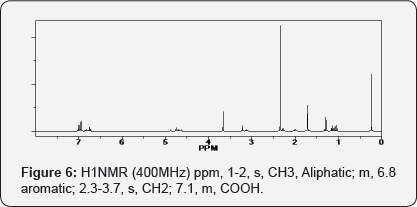
Aceclofenac-Thymol mutual prodrug, IR (KBr): 3395.22 cm-1 (N-H stretching), 3071.19 cm-1(aromatic, C-H stretching), 1646.40 cm-1 (N-H bending), 751.28, 833.92 (Chloride), 1646.40 (amide) 1286.28 cm-1 (C-N stretching, amine) (Figures 6 & 7).
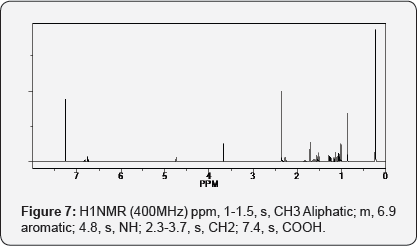
Results and Discussion
The structure was confirmed by IR & NMR (1H NMR) data. The purity of the compounds was checked by TLC. For the preparation of TLC plates, Silica gel G & Silica gel GF were used as a stationary phase, Methanol: CHCl3 (2.5mL: 7.5mL) as a mobile state. Compounds were characterized by melting point. Synthesized compounds were subjected to pharmacological screening. For antimicrobial activity Ciprofloxacin, for antifungal Fluconazole & for anti-inflammatory activity Diclofenac Sodium was used as a standard drug.
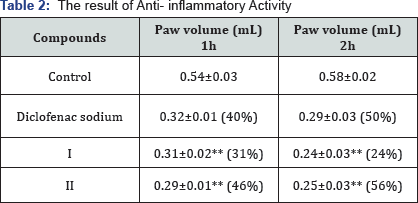
I.The result of Anti- inflammatory Activity
In this study, both compounds showed the similar effectiveness towards the standard drug. Moreover, compound II exhibited the more protection in inflammation remaining another compound of this series (Table 2). The mean Paw volume of anti-inflammatory activity of tested compounds data represents mean ± SEM of two animals per groups and data were analyzed by using one-way ANOVA followed by dinettes**p<0.01 compared to control.
II.The result of Antimicrobial Activity
Antimicrobial activity of both titled compounds showed the better. Still, compound N1 showed the better zone inhibition as compared the standard drug (Table 3).

Conclusion
A mutual prodrug is a form of drug where two pharmacologically active agents are attached to each other in such a way that acts as a promoiety/carrier for each other. In this study, titled compounds act as a mutual prodrug, both compounds showed the significantly anti-inflammatory effect as well as antimicrobial activity. These would be used fully for the treatment inflammation. Further, the study would be concluded the mechanism of action and in-vivo study to predict the efficacy of these compounds on rats towards anti-inflammatory effect.
References
- Laine L (2004) Proton pumps inhibitor co-therapy with nonsteroidal anti-inflammatory drugs-nice or necessary? Rev Gastroenterol Disord 4(suppl 4): S33-S41.
- Scheiman J M (2005) Nonsteroidal anti-inflammatory drugs, aspirin, and gastrointestinal prophylaxis: an ounce of prevention. Rev GastroenterolDisord 5(suppl 2): S39-S49.
- Singh G, Triadafilopoulos G (1999) Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol 56(suppl): 18-24.
- AshutoshKar (Ed.) (2007) Medicinal Chemistry (4th Ed). New Age International Publishers, New Delhi, India, 522-535.
- Roche V F (2009) A Receptor-Grounded Approach to Teaching Non steroidal Anti-inflammatory Drug Chemistry and Structure-Activity Relationships. American Journal of Pharmaceutical Education 73(8): 143.
- Katzung B G (Ed.) (2004) Basic and clinical pharmacology, (9th ed). McGraw-Hill, New York, USA.
- Hardman J G (2001) Limbird L E and Molinoff P B (Eds): Goodman and Gilman's The Pharmacological Basis of Therapeutics, (10th Ed), McGraw-Hill, New York, USA, 689.
- Hawkey C J (1999) COX-2 inhibitors. Lancet 353: 307-314.
- Dogne J M, Supuran C T, Pratico D (2005) Adverse cardiovascular effects of coxibs. J Med Chem 48: 2251-2257.
- Schnitzer T J (2001) COX-2-specific inhibitors: are they safe? J Am Med 110: 46-49.
- Demir S, Yilmaz M, Koseoglu M (2003) Role of free radicals in peptic ulcer and gastritis. Turk J Gastroenterol 14(1): 39-43.
- Mandal S, Yadav S, Nema R (2009) Antioxidants: A Review. Journal of Chemical and Pharmaceutical Research 1(1): 102-104.
- Nakatani M (2000) Phenolic antioxidants from herbs and spices. Biofactors 13: 141-146.
- Repetto M G, Llesuy S F (2002) Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Br J Med Biol Res 35: 523-534.
- Bhosle D, Bharambe S, Garrola N, Dhaneshwar S S (2006) Mutual prodrug concept: fundamentals and applications. Indian J Pharma Sci 68: 286-294.
- Leppanen J, Huuskonen J, Nevalainen T, Gynther J, Taipal H, et al. (2002) Design and synthesis of a novel L-dopa entacaponecodrug. J Med Chem 45:1379-1382.
- Wermuth CG, Ganellin CR, Lindberg P, Mitscher L A (1998) Glossary of terms used in Medicinal Chemistry. Pure and Applied Chemistry 70: 1129-1143.






























