Biological Applications of Quinazolinone Analogues: A Review
K. P. Rakesh1*, N. Darshini2, T. Shubhavathi2 and N. Mallesha2
1Department of Pharmaceutical Engineering, Wuhan University of Technology, China
2SRI RAM CHEM, R & D Centre, India
Submission: April 10, 2017; Published: April 19, 2017
*Corresponding author: K P Rakesh, Department of Pharmaceutical Engineering, Wuhan University of Technology, School of Chemistry, Chemical Engineering and Life Science, 205 Luoshi Road, Wuhan, 430073, PR China, Tel: 86 13007173116; Email: rakeshasg@gmail.com
How to cite this article: K. P. Rakesh, N. Darshini, T. Shubhavathi, N. Mallesha. Biological Applications of Quinazolinone Analogues: A Review. Organic & Medicinal Chem IJ. 2017; 2(2): 555585. DOI: 10.19080/OMCIJ.2017.02.555585
Abstract
Quinazolines and quinazolinones are among the most useful heterocyclic compounds from both synthetic and medicinal chemistry aspects. The structural design of these scaffolds has attracted a great deal of attention because of their ready accessibility, diverse chemical reactivity, and broad spectra of biological activities. Although, the literature is enriched with numerous examples of these motifs exhibiting potential biological activities, we highlighted here the most recent developments in the activity profile of these compounds.
Keywords: Quinazolinones analogues; Biological applications
Introduction
Nitrogen-containing heterocyclic compounds are the most abundant and integral scaffolds that occur ubiquitously in a variety of synthetic drugs, bioactive natural products, pharmaceuticals and agrochemicals. Owing to their widespread applications, these skeletons have long been a subject of immense interest, and substantial efforts have been made to the development of synthetic strategies which could lead to the discovery of new bioactive compounds in medicinal chemistry [1]. Indeed, with particular reference to the pharmaceutical industry, heterocyclic motifs are especially prevalent with over 60% of the top retailing drugs containing at least one heterocyclic nucleus as part of the overall topography of the compound [2]. The presence of N-heterocycles as an essential structural motif in a variety of biologically active substances has stimulated the development of new strategies and technologies for their synthesis.
Among the N-containing heterocycles, quinazolinone is a building block for approximately 200 naturally occurring alkaloids isolated to date from a number of families of the plant kingdom, from animals and from microorganisms. The first quinazoline alkaloid to be isolated was vasicine (peganine 1) in 1888, produced by Indian medicinal tree Adhatoda vasica and later isolated from other species along with the quinazolinone alkaloids, vasicinone 2 and deoxyvasicinone 3 [3]. A variety of other quinazoline and quinazolinone natural products have been isolated, characterized and synthesized thereafter. The first quinazolinone was synthesized in the late 1860s from anthranilic acid and cyanogens to give 2-cyanoquinazolinone 4 [4].
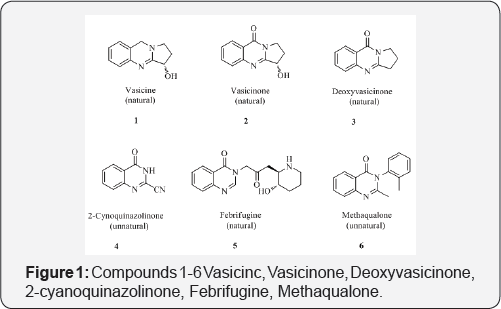
Interest in the medicinal chemistry of quinazolinone derivatives was stimulated in the early 1950 with the structural elucidation of a quinazolinone alkaloid,3-[β-keto-γ-(3- hydroxy-2-piperidyl)-propyl]-4-quinazolone (febrifugine, 5) [5] from an Asian plant Dichroa febrifuga, which is an ingredient of a traditional Chinese herbal remedy, effective against malaria. In a quest to find additional potential quinazolinone-based drugs, various substituted quinazolinones have been synthesized, which led to the synthesis of the derivative, 2-methyl-3-o- tolyl-4-(3H)-quinazolinone (methaqualone 6). Methaqualone was synthesized for the first time in 1951 and it is the most well- known synthetic quinazolinone drug, famous for its sedative- hypnotic effects [6] (Figure 1).
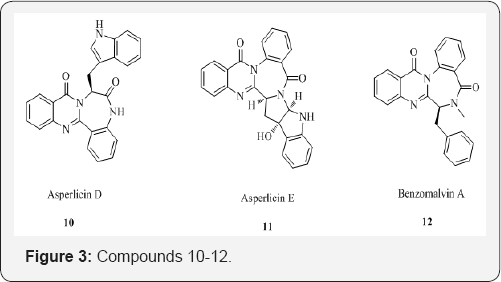
The introduction of methaqualone and its discovery as a hypnotic triggered the research activities toward the isolation, synthesis and studies on the pharmacological properties of the quinazolinones and related compounds. Quinazoline and quinazolinone alkaloids are one of the attractive natural products leading to drug developments. Bioassay-directed isolation followed by identification and characterization of bioactive compounds leads to the development of new medicinal drugs. The structural diversity of quinazolinones has been broadened with the discovery of asperlicin along with asperlicins B, C, D, and E (7-11) produced by Aspergillus alliaceus, which is a potent cholecystokinin (CCK) antagonist. A series of new quinazoline alkaloids fused with benzodiazepinone (3) were also isolated from a fungus culture of Penicillium sp., wherein benzomalvin A 12 is prototypical member. A brief survey on the biological activities of quinazolinone derivatives showed anti-inflammatory [7,8], antitumor [9,10], anti HIV [11], antimicrobial [12,13], CNS depressant and anticonvulsant activities [14,15], antimalarial [16], antitubercular [17], antiviral [18], antihypertensive [19], antidiabetic [20] and cholinesterase inhibition [21]. This broad spectrum of activities has been further facilitated by the synthetic versatility of quinazolinones which allows the generation of a large number of structurally diverse molecule [22] (Figure 2).

Biological Importance of Quinazolinone Analogues
Antimicrobial activity
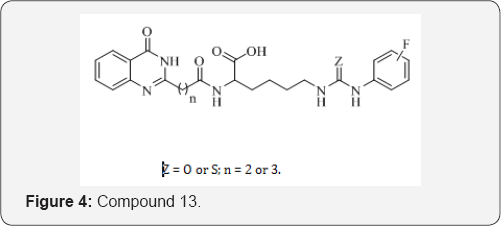
G. P. Suresha et al. [23] reported a novel series of urea/ thioureas of quinazolinones conjugated lysine 13 and screened for their in vitro antimicrobial activity. The activity results showed that the compounds containing urea and thiourea derivatives exerted highly potent activity compared to standards. Further, it is interesting to note that fluoro group attached to the phenyl ring of the conjugates acts as an active moiety in arresting the growth of the microbes. Thus the nature of the substituent was found to be crucial to improve the activity (Figure 3).
M. Rana et al. [24] reported a series of 1-[2-(6-nitro-4- oxo-2-phenyl-4H-quinazolin-3-yl)-ethyl]3-phenyl ureas. The synthesized compounds were assayed for antimicrobial activity by serial broth dilution technique. Some of the compounds showed good activity against different bacterial strains. Good inhibition was observed for compound 14 against strain E. coli at 40 μg/mL of MIC. Similarly for antifungal activity, compound 14 was found to be the most active against the strain C. albicans showing inhibition potency at 10 μg/mL of MIC and 11 mm of zone of inhibition at 20 μg/mL and 20 mm zone of inhibition at 300μg/mL (Figure 4).
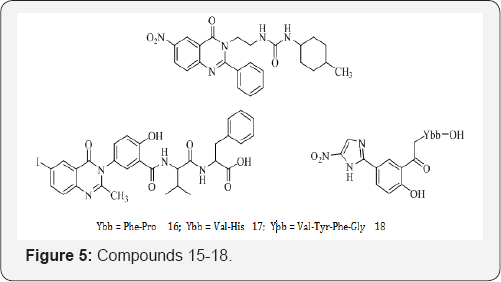
R. Dahiya et al. [25] have synthesized two substituted quinazoline/imidazolyl-salicylic acid conjugated with amino acid/peptides and were assayed for antimicrobial and anthelmintic activities against eight pathogenic microbes and three earthworm species. Among the tested compounds, 15 and 16 exhibited higher antimicrobial activity against Pseudomonas aeruginosa, Klebsiella pneumoniae and Candida albicans and 17 displayed better antifungal activity against the dermatophytes Trichophyton mentagrophytes and Microsporum audouinii. Moreover, 18 showed good anthelmintic activity against Megascoplex konkanensis, Pontoscotex corethruses and Eudrilus eugeniea at a dose of 2 mg mL-1 (Figure 5).
Anti-inflammatory activity
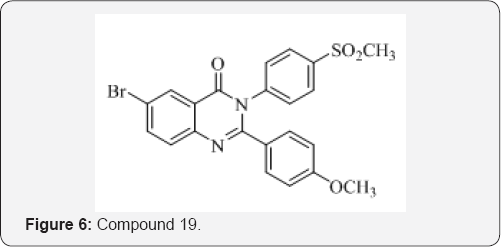
E. Manivannan and S. C. Chaturvedi [26] have synthesized a series of methyl sulfanyl/methyl sulfonyl substituted 2,3-diaryl quinazolinone derivatives. These compounds were evaluated for both non-ulcerogenic and anti-inflammatory activities. The compound 19 emerged as the most active compound in this series. Halogen atoms or methoxy groups at R1 and R3 position of 2,3-diaryl-3H-quinazolin-4-ones appears to have a positive effect on the non-ulcerogenic and anti-inflammatory potency (Figure 6).
Antitumor activity
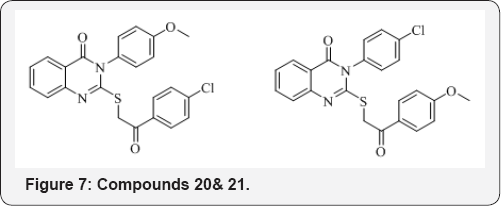
M. A. G. Nagwa et al. [27] synthesized some new 3-substituted quinazolin-4(3H)-ones and 3, 4-dihydro-quinazolin-2(1H)-one derivatives and reported that compounds 20 and 21 as broad spectrum antitumors showing effectiveness toward numerous cell lines that belong to different tumor subpanels (Figure 7).
Antimalarial activity
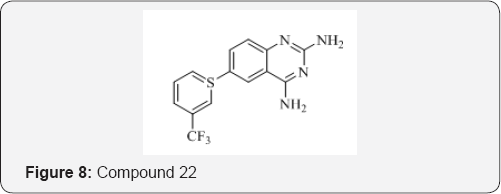
L. M. Werbel et al. [28] synthesized a variety of analogues of 2,4- diamino-6-[(aryl)thio] quinazolines with known antimalarial properties wherein the 4-amino group was replaced by hydrazine and hydroxyamino moieties and they found that such changes reduce markedly the antimalarial properties of this series. The compound 22 was tested against a normal drug- sensitive strain of Plasmodium berghei in mice by the parenteral route (Figure 8).
Antioxidant activity
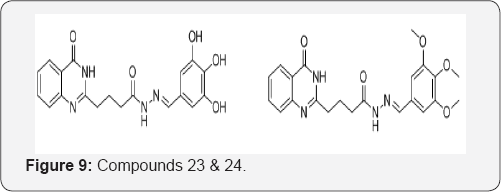
Rakesh et al. [29], designed and synthesized a series of quinazolinone derived Schiff's bases and screnned for their antioxidant activity. Compounds 23 and 24 with OH and OCH3 groups in benzene ring (electron donating) exhibited stronger radical scavenging activities than the standards (Figure 9).
Antiproliferative activity
S. L. Cao et al. [30] reported a novel series of 4-substituted-piperazine-1-carbodithioate derivatives of 2,4-diaminoquinazoline were tested for their antiproliferative activities against five human cancer cell lines including A549 (lung cancer), MCF-7 (breast adenocarcinoma), HeLa (cervical carcinoma), HT29 and HCT-116 (colorectal cancer). Among the synthesized compounds 25-27 were the most active members with IC50 values in the range 1.58-2.27, 1.84-3.27 and 1.47-4.68 μM against five cancer cell lines examined, respectively (Figure 10).

Antituberculosis activity
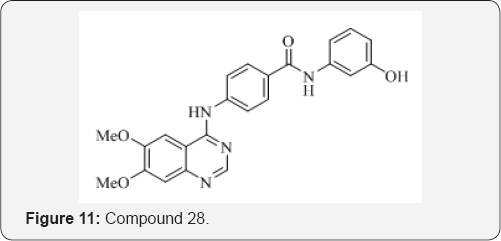
A. T. Tran et al. [31] synthesized a series of quinazoline derivatives and evaluated against M. tuberculosis as GlmU uridyltransferase inhibitors. The most potent inhibitor 28 in this series exhibited an IC50 of 74 μM against GlmU uridyltransferase more potent inhibitors (Figure 11).
Analgesic activity
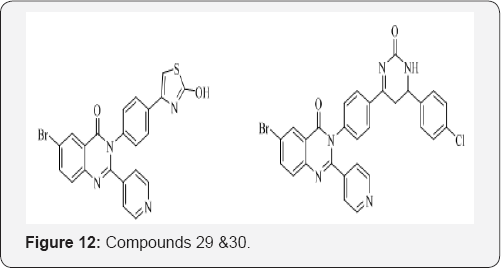
F. Eweas et al. [32] designed and synthesized some novel 2-pyridyl(3H)quinazolin-4-one derivatives and evaluated for their analgesic activity. All the tested compounds showed good analgesic activity in comparison to the reference standard indomethacin. The percentages of maximal protection were noted with the tested compounds 29 and 30 by 75.67 and 75.40 %, respectively (Figure 12).
Antihypertensive activity
V. Alagarsamy and U. S. Pathak [33] synthesized a series of 3-benzyl-2-substituted-3H-[1,2,4]triazolo[5,1-b]quinazolin-9- ones and evaluated for their in vivo antihypertensive activity using spontaneously hypertensive rats (SHR). While all the test compounds exhibited significant antihypertensive activity, compound 31 was found to be the most active antihypertensive agent than the reference standard prazocin (Figure 13).

Rakesh et al. [34], designed and synthesized a series of quinazolinone derived Schiff's bases and screened for their in vitro antiulcer activity. Compounds 32 and 33 with OH and OCH3 groups in benzene ring (electron donating) showed excellent antiulcer activity than the standards (Figure 14).
Conclusion
The study of natural and synthetic quinazolinones used as potential therapeutic agents. However over the past two decades there has been a assault into searching better drugs with minimal side effects. There is scope for more development as new research into study of medicinal chemistry related pathways, as is observed by the report of biological evaluation and molecular docking studies reported in recent years. Here, we make a comprehensive record of the study of quinazolinones used as biological applications and also hope that it will help the workers to further their work as a point of reference.
Acknowledgements
We are grateful to Wuhan University of Technology, Wuhan, China for financial support, the authors are also thankful to SRI RAM CHEM (India) management for their continuous encouragement towards the research.
References
- V Polshettiwar, R S Varma (2007) Greener and sustainable approaches to the synthesis of pharmaceutically active heterocycles. Curr Opin Drug Discov Dev 10(6): 723-737.
- NA McGrath, M Brichacek, J T Njardarson (2010) A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J Chem Educ 87: 1348-1349.
- SEguchi (2006) Top Heterocycl Chem 6: 113-156.
- P Griess (1869) Ber 2: 415.
- J B Koepfly, J F Mead, J A Brockman (1947) An Alkaloid With High Antimalarial Activity From Dichroa Febrifuga. J Am Chem Soc 69(7): 1837.
- I K Kacker, S H Zaheer (1951) Synthesis of Substituted 4-Quinazolones. J Indian Chem Soc 28: 344-346.
- PM Chandrika, T Yakaiah, A R Rao, B Narsaiah, N C Reddy, et al. (2008) Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur J Med Chem 43: 846-852.
- RS Giri, H M Thaker, T Giordano, J Williams, D Rogers, et al. (2009) Eur J Med Chem 44: 2184-2189.
- HJ Park, Y S Kim, J S Kim, E J Lee, Y J Yi, et al. (2004) Bioorg Med Chem Lett 14: 3385-3388.
- YJin, Z Y Zhou, W Tian, Q Yu, Y Q Long (2006) Bioorg Med Chem Lett 16: 5864-5869.
- V Alagarsamy, S Murugesan, K Dhanabal (2007) AntiHIV, antibacterial and antifungal activities of some novel 2-methyl-3-(substituted methylamino)-(3H)-quinazolin-4-ones. Indian J Pharm Sci 69: 304307.
- KP Rakesh, R Suhas, HM Manukumar, S Chandan, D Channe Gowda (2015) Quinazolinones linked amino acids derivatives as a new class of promising antimicrobial, antioxidant and anti-inflammatory agents. Eur J Chem 6: 254-260.
- NB Patel, G G Barat (2010) J Saudi Chem Soc 14: 157-164.
- H Georgey, N Abdel-Gawad, S Abbas (2008) Synthesis and Anticonvulsant Activity of Some Quinazolin-4-(3H)-one Derivatives. Molecules 13(10): 2557-2569.?
- SK Kashaw, V Kashaw, PMishra, N K Jain, JP Stables (2009) Eur J Med Chem 44: 4335-4343.
- S Jiang, Q Zeng, M Gettayacamin, A Tungtaeng, S Wannaying, et al. (2005) Antimalarial Activities and Therapeutic Properties of Febrifugine Analogs. Antimicrob Agents Chemother 49: 1169-1176.
- S M Mosaad, K I Mohammed, M A Ahmed, SG Abdel-Hamide (2004) Synthesis of Certain New 6-Iodoquinazolines as Potential Antitubercular Agents. J Appl Sci 4: 302-307.
- MA Saleh, M F Abdel-Megged, M A Abdo, A M Shokr (2002) Synthesis And Antiviral Evaluation Of Some New Glycosylthioureas Containing A Quinazolinone Nucleus. Nucleosides Nucleotides Nucleic Acids 21: 93-106.
- VK Pandey, S K Bajpai (2004) Thiadiazolyl quinazolones as potential antiviral and antihypertensive agents. Indian J Chem 43B: 180-183.
- MS Malamas, J Millen (1991) Quinazolineacetic acids and related analogs as aldose reductase inhibitors. J Med Chem 34: 1492-1503.
- M Decker (2005) Eur J Med Chem 40: 305-313.
- Rashmi, K Ashish, N S Gill, A C Rana (2011) Quinazolinone: An Overview. I R J P 2: 22-28.
- G P Suresha, K C Prakasha, K N Shivakumara, K Wethroe, D C Gowda (2009) Design and Synthesis of Heterocyclic Conjugated Peptides as Novel Antimicrobial Agents. Int J Pept Res Ther 15: 25-30.
- M Rana, K R Desai, S Jauhari (2013) Synthesis, characterization, and pharmacological evaluation of 1-[2-(6-nitro-4-oxo-2-phenyl-4H- quinazolin-3-yl)-ethyl]-3-phenyl ureas. Med Chem Res 22: 225-233.
- R Dahiya, A Kumar, R Yadav (2008) Synthesis and biological activity of peptide derivatives of iodoquinazolinones/nitroimidazoles. Molecules 13: 958-976.
- E Manivannan, S C Chaturvedi (2011) Bioor Med Chem 19: 4520-4528.
- M A G Nagwa, HG Hanan, M Y Riham, A E S Nehad (2010) Eur J Med Chem 45: 6058-6067.
- L M Werbel, MJ Degnan (1987) Antimalarial drugs. 63. Synthesis and antimalarial and antitumor effects of 2-amino-4-(hydrazino and hydroxyamino)-6-[(aryl)thio]quinazolines. J Med Chem 30: 21512154.
- K P Rakesh, H M Manukumar, D Channe Gowda (2015) Bioorg Med Chem Lett 65: 1072-1077.
- S L Cao, Y Han, C Z Yuan, Y Wang, Z K Xiahou, et al. (2013) Eur J Med Chem 64: 401-409.
- A T Tran, W Daying, P Nicholas, N B Edward, W J Brittonc, R J Payne (2013) Inhibition studies on Mycobacterium tuberculosis N-acetylglucosamine-1-phosphate uridyltransferase (GlmU). Org Biomol Chem 11: 8113-8126.
- F Eweas, A O H El-Nezhawy, A R Baiuomy, M M Awad (2013) Design, synthesis, anti-inflammatory, analgesic screening, and molecular docking of some novel 2-pyridyl (3H)-quinazolin-4-one derivatives. Med Chem Res 22: 1011-1020.
- V Alagarsamya, U S Pathak (2007)Bioor Med Chem 15: 3457-3462.
- K P Rakesh, CS Shantharam, H M Manukumar (2016) Bioorg Chem 68: 1-8.






























