Simple Synthesis of Fluoroorganic Compounds
Mieczyslaw Mąkosza*
Institute of Organic Chemistry, Polish Academy of Sciences, Europe
Submission: March 31, 2017; Published: April 05, 2017
*Corresponding author: Mieczyslaw Mąkosza, Institute of Organic Chemistry, Polish Academy of Sciences, ul. Kasprzaka 44/52, 01-224 Warszawa, Poland, Europe, Email: icho-s@icho.edu.pl
How to cite this article: Mąkosza M. Simple Synthesis of Fluoroorganic Compounds. Organic & Medicinal Chem IJ. 2017; 2(2): 555582. DOI: 10.19080/omcij.2017.02.555582
Mini Review
Introduction of fluorine into organic molecules changes their physical, chemical and biological properties, often in desired way, hence most of the new pharmaceuticals contain fluorine [1]. It is therefore evident that methods of synthesis of fluoroorganic compounds are of continuous great interest. There are two major approaches to this goal: introduction of fluorine atom via replacement of hydrogen or other substituents in a molecule and introduction of fluorine containing substituents.
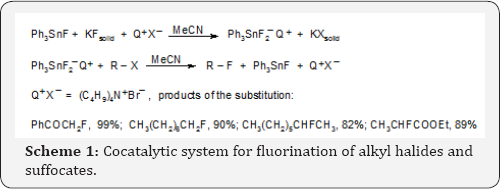
Fluorine can be introduced into organic molecules via nucleophilic, electrophilic and radical mechanisms. Particularly important and simple is nucleophilic replacement of halogens or sulfonates by fluorine anions readily available in form of KF. The most convenient and efficient methodology for nucleophilic substitution with inorganic and organic anions is phase-transfer catalysis, PTC. The key feature of this methodology is continuous introduction of the reacting anions into organic solvents in form of lipophilic ion-pairs with lipophilic cations of the catalyst, most often tetraalkyl ammonium cations [2,3]. Unfortunately use of this efficient methodology for the reactions of fluorine anions encounters difficulties, because they are of low lipophilicity. Moreover fluoride anions are highly basic, hence nucleophilic substitution is often accompanied by base induced β-elimination E2 that dominates in the case of secondary halides or sulfonates. These difficulties are solved by use of triphenyl tin fluoride as a cocatalyst in phase transfer catalyzed fluorination. The modus operandi of the cocatalytic system is shown in Scheme 1 [4].
Triphenyl tin fluoride (or chloride) reacts with solid powdered KF and tetraalkyl ammonium halide (Q+X) in acetonitrile to form soluble lipophilic ion-pairs of hipervalent triphenyltin difluoride anion with tetraalkylammonium, Q+cation. In this form this hipervalent anion reacts with alkyl halides or sulfonates to form alkyl fluorides, triphenyl tin fluoride and Q+X . This catalytic cycle proceeds many times, so less than 5% molar of the both catalysts are sufficient for full conversion of alkyl halides or sulfonates into fluorides. Since the substitution proceeds with fluorides that are in form of hypervalent tin anions, they do not behave as basic agent and undesired β-elimination does not proceed [4].
Of particular importance is introduction of fluorinated or per fluorinated substituents into carbo- and heterocyclic aromatic rings [5]. Most of such reported processes are catalyzed by transition metals hence are of limited use for synthesis of pharmaceuticals.
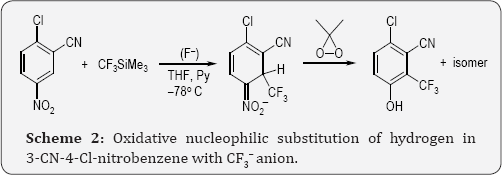
For many years we have developed efficient procedures for introduction of functionalized substituents into electron- deficient arenes and heteroarenes via nucleophilic substitution of hydrogen - oxidative nucleophilic substitution ONSH and vicarious nucleophilic substitution VNS [6]. Application of these reactions for perfluorocarbanions, e.g. for CF3 , generated from the Ruppert reagent, CF3SiMe3 was limited due to low nucleophilic ity of this anion. Nevertheless in the reaction with highly active nitroarenes e.g. m-cyan nitrobenzene or m-dinitrobenzene ONSH with CF3~ carbanions proceed, Scheme 2 [7].
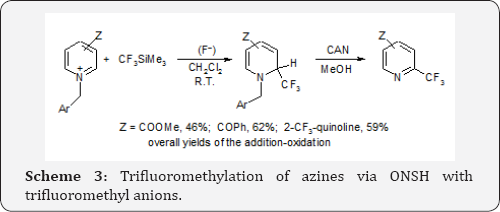
Much more efficient and valuable are reactions of perfluorocarbanions with highly active azinium salts. Thus N-benzyl and particularly N-p-methoxybenzylpyridinium, quinolinium, isoquinolinium etc. salts when treated with the Ruppert reagent in the presence of F- anions CF- carbanions to form stable dihydroazines. Further oxidation with cerium ammonium nitrate, CAN, results in rearomatisation and removal of the benzyl substituents to give trifluoromethylazines, Scheme 3 [8]
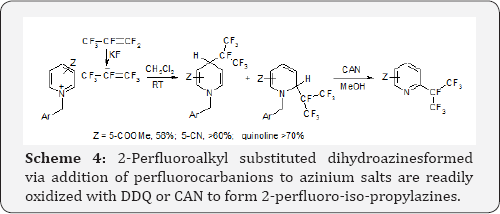
Perfluoroalkyl carbanions can be generated via addition of F- anions to perfluoroalkenes. For instance exposition of readily available perfluoropropene to anhydrous KF in methylene dichloride results in reversible addition of F- anions, thus some amount of potassium perfluoro-isopropyl carbanion is generated in the solution. The anion adds to N-benzyl azinium salts to form stable perfluoro-iso-propyl dihydroazines. Due to large size of this carbanion the addition proceeds at positions 2-(ortho) and 4- (para) of the salts. Oxidation of these dihydroazines results in rearomatization and removal of the N-benzyl substituents Scheme 4 [9].
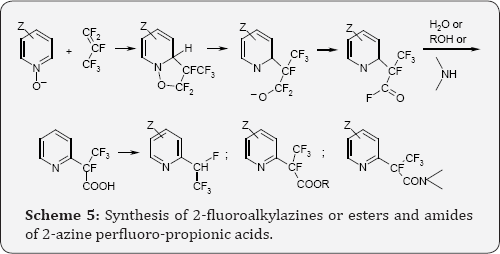
Particularly valuable are syntheses of esters, amides and other derivatives of 2-azine-perfluoropropionic acids via 1,3-dipolar cycloaddition of azine-N-oxides to perfluoroalkenes. The addition results in formation of unstable isoxazolidines which rearomatize via dissociation of O-N bond in the isoxazolidines ring. Subsequent departure of fluoride anions provides acid fluorides. Treatment of the reaction mixture with water results in hydrolysis and instantaneous decarboxylation to form 2-fluoroalkylated azines. On the other hand in situ treatment of the produced acyl fluorides with alcohols, amines, etc. gives esters, amides etc. of 2-azine perfluoro-propionic acids, Scheme 5 [10].
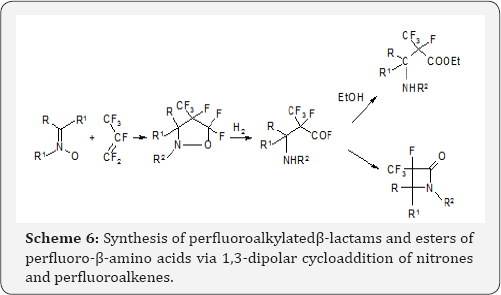
Azine N-oxides are analogues of aliphatic nitrones, thus nitrones can also enter 1,3-dipolar cycloaddition to perfluoroalkenes to form isoxazolidines. These isoxazolidines are sufficiently stable to be isolated. Upon hydrogenation N-O bond is broken to form fluorides of fluorinated β-aminoacids. These fluorides cyclized in situ giving β-lactams. When the hydrogenation is carried out in acidic medium, in alcohols, esters of fluorinated β-aminoacids are produced Scheme 6, [11].

α-Fluoro-α-aryl acetic acid esters are interesting and versatile starting materials for further synthesis. Usually they are prepared in a complicated way [12,13]. Much simpler synthesis of this kind of compounds is vicarious nucleophilic substitution of hydrogen in nitroarenes and nitroheteroarenes with carbanions of alkyl chlorofluoroacetates generated by simple deprotonation of the commercial product,Scheme 7 [14].
In the paper are presented simple processes for synthesis of a wide variety of fluoroorganic compounds. It should be stressed that the presented reactions proceed without transition metal catalysts, thus the products can be directly used for synthesis of pharmaceuticals, without meticulous purification.
References
- Zhou Y, Wang J, Gu Z, Wang S, Zhu W, et al. (2016) New Generation of Fluorine - Containing Pharmaceuticals in Phase II - III Clinical Trials of Major Pharmaceuticals Companies: New Structural Trends and Therapeutical Areas. Chem Rev 116(2): 422-518.
- MąkoszaM, Fedorynski M (2003) Phase Transfer Catalysis. Catalysis Reviews 45: 321-361.
- Dehmlow E V, Dehmlow S S (1993) Phase Transfer Catalysis. Third Edition VCH Weinheim.
- Mąkosza M, Bujok R (2005) Cocatalysis in phase-Transfer Catalyzed Fluorination of Alkyl Halides and Sulfonates. J Fluorine Chem 126: 209-216.
- Alonso C, Martines de Marigota E, Rubiales G, Palacios F (2015) Carbon Trifluor ometylation Reactions of Hydrocarbon Derivatives and Heteroarenes. Chem Rev 115(4): 1847-1935.
- Mąkosza M, Wojciechowski K (2004) Nucleophilic Substitution of Hydrogen in Heterocyclic Chemistry. Chem Rev 104(5): 2631-2666.
- Surowiec M, M^kosza M (2004) Oxidative Nucleophilic Substitution of Hydrogen in Nitroarenes with Trifluoromethyl Carbanions, Synthesis of Trifluoromethyl Phenols. Tetrahedron 60: 5019-5024.
- Loska R, Majcher M, Mąkosza M (2007) Synthesis of TrifluoromethylatedAzines via Nucleophilic Oxidative Substitution of Hydrogen by Trifluoromethyl Carbanions. J Org Chem 72 (15): 55745580.
- Loska R, Mąkosza M (2007) Synthesis of Perfluoroalkyl - Substituted Azines via Nucleophilic Substitution of Hydrogen with Perfluoroisopropyl Carbanions. J Org Chem 72(4): 1354-1365.
- Loska R, Mąkosza M (2008) New Synthesis of 2-Heteroarylperfluoropropionic Acids Derivatives by Reaction of Azine- N-Oxides with Hexafluoropropene. Chem Eur J 14(8): 25772589.
- Jakowiecki J, Loska R, Mąkosza M (2008) Synthesis of a-Trifluoromethyl- P-lactams and Esters of p-Amino Acids via 1,3-Dipolar Cycloaddition of Nitrones to Fluoroalkenes. J Org Chem73 (14): 5436-5441.
- Su YM, Feng GS, Wang ZY, Lau Q, Wang XS (2015) Nickel Catalyzed Monofluoromethylation of Aryl Boronic Acids. Angew Chem 54 (20): 6003-6007.
- Harsanyi A, Sandford G, Yufit D S, Howard J A K (2014) Syntheses of fluorooxindole and 2-fluoro-2-arylacetic acid derivatives from diethyl 2-fluoromalonate ester. Beilstein J Org Chem 10: 1213-1219.
- Czaban-Jozwiak J, Loska R, Mąkosza M (2016) Synthesis of a-Fluoro-a- nitroarylacetates via Vicarious Nucleophilic Substitution of Hydrogen. J Org Chem 81 (23): 11751-11757.






























