Phytochemical and Antioxidant Activities of Ipomea Ochraceae
Idowu Olajumoke Tolulope*
Department of Chemistry, Afe Babalola University, Nigeria
Submission: March 07, 2017; Published: March 17, 2017
*Corresponding author: Idowu Olajumoke Tolulope, Department of Chemistry, College of Chemical Science, Afe Babalola University, Ado -Ekiti, Ekiti State, Nigeria, Tel: +2348037400826; Email: jumokeye@gmail.com
How to cite this article: Idowu O T. Phytochemical and Antioxidant Activities of Ipomea Ochraceae. Organic & Medicinal Chem IJ. 2017; 2(1): 555577. DOI: 10.19080/OMCIJ.2017.02.555577
Abstract
lpomeaochraceae whole plant was alr-drled and extracted using ethanol. The purpose of this study was to determine the presence of phytochemlcalsand also to evaluate the antloxldant activity using DPPH and FRAP.
Keywords: DPPH; lpomeaochraceae; FRAP; Antloxldants
Mini Reveiw
The family Convolvulaceae includes about 57 genera and 1625 species Shimpak VB [1]. lpomoea is one of the dominant genera within this family with approximately 650 species, mainly distributed in tropical and warm temperate regions of the world and known as "morning glories" Mabberley DJ [2]. Most of the species within this genus are twining climbing plants and include annual and perennial herbs, lianas, shrubs and small trees Mabberley DJ [2]. The genus includes important food crops (sweet potato and water spinach) and Ipomeao violacea, source of the Mexican psychedelic drug,tlitliltzin Species and cultivars of lpomoea grown as morning glory are popular in gardens for their often stunning flowers.Ipomeaochracea is popularly called yellow morning glory. lt is referred to as weed. Little work has been done on this plant because it is referred to as weed and of no importance to both human and animal health. The present work focus on the whole plant to test for phytochemicals and antioxidant activity.
Materials and Methods
Plant Material and General Experimental Methods
Fresh leaves of Ipomeaochracea were collected in the month of November, 2016 from AfeBabalola University, Ado-Ekiti (ABUAD)Ekiti state, Nigeria. The plant was authenticated by the herbarium staff of the Botanical department at the Ekiti State University.
I. Sample preparation: 950g of the leaves were air dried at room temperature for about seven days. Thereafter, the dried leaves were pulverized and extracted using 95% ethanol at room temperature. The extracts were concentrated to dryness and the residue were obtained as a greenish black solid, after which the residues were transferred into a pre-weighed sample containers and were stored and later used for phytochemical screening and antioxidants activity.
II. Phytochemical screening: The leaves extract of were analyzed for the presence of alkaloid, saponin, anthraquinone, steroids, tannin, flavonoid, reducing sugars and cardiac glycosides according to standard methods Odebiyi and Sofowora [3].
III. Qualitative phytochemical analysis: Qualitative phytochemical analysis of aqueous extracts of was carried out on the extract using standard procedure to identify the constituents as described by Sofowora [4], Trease and Evans and Harbone [5,6].
IV. Test for Taninns: 1ml of extract was boiled in 20ml of water in a test tube and then filtered. A few drops of 0.1% ferric chloride was added and observed green or a blue - black coloration which confirms the presence of tannin.
V. Test for Phlobatanins: Deposition of a red precipitate when 2ml of extract of each plant samples was boiled with 1% aqueous hydrochloric acid was taken as evidence for the presence of phlobatanins.
VI. Test for Saponin: About 5ml of the extract was boiled in 20ml of distilled water in a water bath and filtered. 10ml of the filtrate was mixed with 5ml of distilled water and shaken vigorously for a stable persistent froth. The frothing was mixed with 3 drops of olive oil and shaken vigorously, then observed for the formation of emulsion which confirms a positive presence of Saponin.
VII. Test for Flavonoids: 3ml of 1% Aluminium chloride solution was added to 5ml of each extract. A yellow coloration was observed indicating the presence of flavonoids. 5ml of dilute ammonia solution was added to the above mixture followed by addition of concentrated H2SO4. A yellow coloration disappeared on standing. The yellow coloration which disappeared on standing indicates a positive test for flavonoids.
VIII. Test for Steroids: 2ml of acetic anhydride was added to 2ml extract of each sample followed by careful addition of 2ml H2SO4. The color changed from violet to blue or green indicate the presence of steroids.
IX. Test for Terpenoids (Salkowski test): 5 ml of each extract was mixed with 2ml of chloroform, and 3ml concentrated H2SO4 was carefully added to form a layer. A reddish brown coloration of the interface was formed to show positive results for the presence of terpenoids.
X. Test for Cardiac Glycosides and Cardenolides (Keller - Killani test): 5 ml of each extracts was treated with 2ml of glacial acetic acid containing one drop of ferric chloride solution. This was underplayed with 1ml of concentrated sulphuric acid. A brown ring at the interface indicates a deoxysugar characteristic of cardenolides which confirms a positive presence of cardenolides. A violet-green ring appearing below the brown ring, in the acetic acid layer, indicates the positive presence of glycoside.
XI. Alkaloids: 1ml of the extract was stirred with 5ml of 1% aqueous HCl on a steam bath and filtered while hot. Distilled water was added to the residue and 1ml of the filtrate was treated with a few drops of either Mayer's reagent (Potassium mercuric iodide- solution) or Wagner's reagent (solution of iodine in Potassium iodide) or Dragendorff^s reagent (solution of Potassium bismuth iodide). The formation of a cream color with Mayer's reagent and reddish-brown precipitate with Wagner's and Dragendorff^s reagent give a positive test for alkaloids.
XII. Phenol: 5ml of the extract was pipetted into a 30ml test tube, and then 10ml of distilled water was added. 2ml of ammonium hydroxide solution and 5 ml of concentrated amyl alcohol was also added and left to react for 30min. Development of bluish green color was taken as a positive presence of phenol.
XIII. Determination of total Phenolic Compounds: 100mg of the extract of the sample was weighed accurately and dissolved in 100 ml of triple distilled water (TDW). 1 ml of this solution was transferred to a test tube, then 0.5 ml 2N of the Folin- Ciocalteul and 1.5ml 20% of Na2CO3 solution was added and ultimately the volume was made up to 8ml with TDW followed by vigorous shaking and finally allowed to stand for 2hours after which the absorbance was taken at 765 nm. These data were used to estimate the total phenolic content using a standard calibration curve obtained from various diluted concentrations of garlic acid Hagerman et al. [7].
Determination of total Flavonoids
The method is based on the formation of the flavonoids-aluminium complex which has an absorptivity maximum at 415 nm. 100 μl of the plant extracts in methanol (10 mg/ml) was mixed with 100 μl of 20 % aluminiumtrichloride in methanol and a drop of acetic acid, and then diluted with methanol to 5 ml. The absorption at 415 nm was read after 40minutes. Blank samples were prepared from 100ml of plant extract and a drop of acetic acid, and then diluted to 5 ml with methanol. The absorption of standard rutin solution (0.5 mg/ml) in methanol was measured under the same conditions. All determinations were carried out in triplicates Kuamaran and Karunakaran [8].
Determination of total Alkaloids
5 g of the sample was weighed into a 250 ml beaker of 10 % acetic acid in ethanol was added and covered and allowed to stand for 4 h. This was filtered and the extract was concentrated on a water bath to one-quarter of the original volume. Concentrated ammonium hydroxide was added drop wise to the extract until the precipitation was complete. The whole solution was allowed to settle and the precipitated was collected and washed with dilute ammonium hydroxide and then filtered. The residue is the alkaloid, which was dried and weighed Harbone [6].
Determination of total tannins
500 mg of the sample was weighed into a 50 ml plastic bottle. 50 ml of distilled water was added and shaken for 1 hr in a mechanical shaker This was filtered into a 50 ml volumetric flask and made up to the mark. Then 5 ml of the filtered was pipette out into a test tube and mixed with 2 ml of 0.1 M FeCl3 in 0.1 N HCl and 0.008 M Potassium ferrocyanide. The absorbance was measured at 120 nm within 10 minutes Van-Burden TP and Robinson T [9].
Determination of total saponin
The samples were powdered and 20 g of each were put into a conical flask and 100 cm3 of 20% aqueous ethanol were added. The samples were heated over a hot water bath for 4 h with continuous stirring at about 55o C. The mixture was filtered and the residue re-extracted with another 200 ml 20% ethanol. The combined extracts were reduced to 40 ml over water bath at about 90oC. The concentrate was transferred into a 250 ml separatory funnel and 20ml of diethyl ether was added and shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated 60ml of n-buthanol was added. The combined n-buthanol extracts were washed twice with 10ml of 5% aqueous sodium chloride. The remaining solution was heated in a water bath.After evaporation the samples were dried in the oven to a constant weight; the saponin content was calculated Obadomi B and Ochuko P [10].
Antioxidants
Antioxidant (Ferric Reducing Antioxidant Power). Prepare 0.5ml of the extract and 0.5 ml of H2O with 2.5ml of phosphate buffer for each sample and add 2.5 ml of potassium ferricyanide and incubate at 50 for 20 minutes. Add Trichloroacetic acid to stop the reaction. Separate reaction mixture in aliquots of 2.5ml, and dilute each with also 2.5ml of distilled water. Add 0.5ml of Ferric chloride solution into each tube. Allow to stand in the dark for 30 minutes for colour development. Read absorbance at 700 nm against a reagent blank.
Scavenging of diphenyl-picrylhydrazyl (DPPH) radicals
The scavenging activity of the extracts was determined based on DPPH-scavenging assay described by Molan et al. [11]. Briefly, 0.01g/100ml of each extract was allowed to react with 0.04g/100ml of 9.9 ml DPPH in 95 % ethanol in a 96- well microplate. The plate was then incubated in dark at room temperature for 30 min and the absorbance (A) was measured at 550 nm using a microplate reader and all determinations were performed in triplicate in two separate experiments. Trolox was used positive control. The antiradical activity was calculated as a percentage of DPPH decolouration relative to a negative control using the following equation: Free-radical scavenging activity 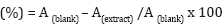
Results
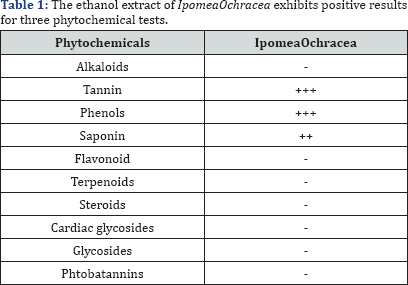
Legend: +++ (Much abundant); ++ (less abundant); + (minute); - (absent)
In qualitative analysis, the ethanol extract of lpomeaOchracea exhibits positive results for three phytochemical tests (Table 1). These phytochemical properties are responsible for medicinal uses in managing,treating and cure somediseases that do affects us. This leads to quantitative analysis of the plant.
Quantitative phytochemical analysis
The amount of phytochemicals which were found in the sample aqueous extract (much abundant {+++} and less abundant {++}) was quantitatively determined by standard procedures. Among the nine compounds present saponin content was the highest 21.50mg/g while phenol content was the lowest 7.80mg/g (Tables 2 & 3).


Discussion
ln quantitative phytochemical analysis, out of nine compounds that were tested three of them tested positive in which are saponin, tannin and phenols. Saponins are beneficial in lowering cholesterol by binding to the bile acids in the body to reduce the absorption of cholesterol from the blood. It also reduces the risk of colon cancer and lower heart attack. Saponincontent in the Ipomeaochracea that usually refer to as a weed is 21.50mg/g.
Atannin (ortannoid)isanastringent,polyphenolicbiomolecule that binds to and precipitates proteins and various other organic compounds including amino acids and alkaloids. The tannins have the property to coagulate proteins and mucosal tissues, by creating an insulating and protective layer that soothes irritation and pain on the skin. Tannins are considered antioxidants and prevent ‘the onset of degenerative diseases such as cancer and cardiovascular disease Molan et al. [11].
Phenols are present in the whole plant of Ipomeaochraceae. Interestingly, most phenolic phytochemical usually consumed in common diets are antioxidants while some in addition, possess antimicrobial and antifungal properties Kang et al. [12]. Recently, phenolic compounds have been linked with the likelihood of preventing and reducing the effects of Alzheimer's disease Dell'Agliet al. [13]. Antioxidants are known to play a key role in reducing cancer cell proliferation.
Conclusion
This findings show that the whole plant extract of Ipomeaochraceaecontains active phytochemical compounds with antioxidant properties and other degenerative diseases potentials whereas it has been known to be weed. This findings has also confirmed it not to be referred to as a weed but a medicinal plant.
References
- Shimpak VB, PR Kshirsagar, Nilesh V Pawar (2012) lpomeaochracea (convolvulacea)- A new record from India. Rheedea 22(2): 99-102.
- Marbberley DJ (2008) The Plant-Book. A portable dictionary of plants, their classification and uses, Third Edition, Cambridge University Press, lndia.
- OdebiyiA, Sofowora AE (1978) Phytochemical screening of Nigeria medicinal plants. Lioydia 41(3): 234-246.
- Sofowora A (1993) Medicinal plants and Traditional Medicine in Africa. Spectrum Books Ltd, Ibadan Nigeria pp: 191-289.
- Trease GE, Evans WC (1989) A textbook of pharmacognosy (13th edn) BacilluereTinall Ltd, London, UK.
- Harbone IB (1973) Phytochemical methods: A guide to modern techniques of plant analysis 2ndedn, chapman and hall, New York, USA, pp: 18-88.
- Hagerman A, Harvey-Mueller I, Marker HPS (2000) Quatification of tannins in the foliage- a laboratory manuel. ed. FAO/lAEA, Vienna, Austria pp: 4-7.
- Kumaran A, Karunakaran RJ (2006) Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chemistry 97: 109-114.
- Van-Burden TP, Robinson T (1981) The biochemistry of alkaloids, 2ndedn. Springer, Heidelberg, Newyork, USA.
- Obadomi B, Ochuko P (2001) Phytochemical studies comparative efficacy of the crude extracts of some homeostatic plants in Edo and Delta states of Nigeria. Global J Pure Appl Sci 8: 203-208.
- Molan AL, Duncan AJ, Banjand T N, McNabb WC (2003) Effect of condensed tannins and sesquiterpene lactones extracted from Chicory on the motility of larvae of deer lungworm and gastrointestinal nematodes. parasitol lnt 52(3): 209-218.
- Kang SW, Hong SI, Kim SW (2005) Identificatin of Aspergilus strain with antifungal activity against phytophthora species. Journal of Microbiology and Biotechnology 15(2): 227-233.
- DellAgli M, Galli GV, Parapiru S, Basilli N, Taramell D, et al. (2008) Anti plasmodial activity of Ailanthus excels. Fitoterapia 79 (2): 112-116.






























