Synthesis, Drug Likeness and Antioxidant Activity of Analogues of L-Arginine
AinaOlayinka*, Afolabi Ezekiel, Odumosu Patricia and Ojerinde Stephen
Department of Pharmaceutical chemistry, Faculty of Pharmaceutical Sciences, University of Jos, Nigeria
Submission: January 23, 2017; Published: March 08, 2018
*Corresponding author: Aina Olayinka, Department of Pharmaceutical chemistry, Faculty of Pharmaceutical Sciences, University of Jos, Nigeria, Tel: 234080 34027488; Email: yinkaaina13@mail.com
How to cite this article: Aina O, Afolabi E, P O Odumosu, Stephen O. Synthesis, Drug Likeness and Antioxidant Activity of Analogues L-Arginine. Organic & Medicinal Chem IJ. 2017; 1(5): 555574. DOI: 10.19080/OMCIJ.2017.01.555574
Abstract
Free radicals damage is responsible for the development many chronic health problems. 3 analogues of L-arginine were synthesized and their structures were confirmed with FTIR, 1H and 13C NMR. Molecular properties and bioactivity prediction of the synthesized compounds was carried out with molinspirationsoftware. The antioxidant activity of the synthesized compounds was evaluated using DPPH radical scavenging assay. The compounds obeyed Lipinsky rule of five and showed good bioactivity scores. The analogues A1-A3 exhibited low antiradical activity against with 1C50 value of 631.06, 501.19 and 537.02^g/ml respectively.
Keywords: L-arginine; DPPH; Antioxidant; Lipinsky; Molinspiration
Introduction
Free radicals are always produced in biological system and also found exogenously and are known to lead to a range of degenerative disorders [1]. Excessive production of free radicals can trigger oxidative damage to biomolecules like lipids, proteins and DNA eventually leading to several chronic diseases such as atherosclerosis, cancer, diabetes, rheumatoid arthritis, post- ischemic perfusion injury, cardiovascular diseases, aging and other degenerative diseases in humans [2,3]. A balance between free radicals and antioxidants is necessary for proper physiologic function [4].
L-Arginine is asemi essential or conditionally essential amino acid in humans. 1t is involved in many metabolic pathways in the human body. 1t acts as a precursor for the production of urea, polyamines, proline, glutamate, creatine and agmatine [5]. Studies have reported that L-arginine acts as free radical scavenger because of its ability to inhibit the activity of prooxidant enzymes [6]. The guanidine group of L-arginine is important for its biological activity some investigators have proposed a direct antioxidant action related to its amino guanidine moiety in L-arginine [7]. While others infer that the alpha amino group of L-arginine is responsible for its antioxidant activity. This work is aimed to synthesize analogues L-arginine by reaction with anhydrides and sacharrin sodium and to assess the antioxidant properties, bioactivity scores and drug likeness of the compounds.
Materials and Methods
Synthesis of compounds
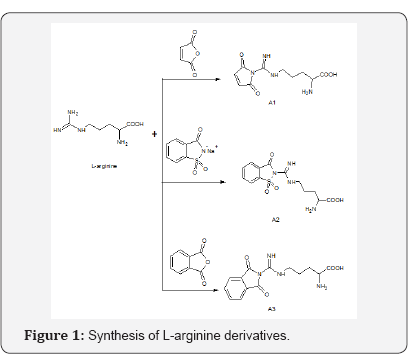
Three analogues of L-arginine were obtained by reacting 0. 02moles of maleic anhydride, sacharrinsodium and phthalic anhydride with 0.02moles of L-arginine (0.02M). The mixture was dissolved in 50 ml absolute ethanol and refluxed for 4 hours until the completion of the reaction (TLC monitoring, using butanol: water; Acetic acid -4:2:2, v/v, UV light at 254 nm) The products (A1, A2 and A3) obtained was allowed to cool at room temperature, decanted into a beaker and allowed to crystallize. The crystals were subsequently weighed and their melting points determined by capillary tube method using the melting point apparatus. The uncorrected melting points of compounds were determined in an open glass capillary using Thomas-Hoover melting point. 1H NMR spectra was recorded with a Bruker AMX- 400Hz Spectrometer in MeOD (deuterated methanol). 13C NMR spectrum was recorded with a Bruker AMX-100Hz Spectrometer in MeOD (deuterated methanol) (Figure 1).
Insilico prediction of bioactivity and drug likeness of synthesized compounds
Structures of the synthesized compounds were drawn using online molinspiration [8] for the calculation of molecular properties such as: (MiLog P, Total polar surface area (TPSA), number of hydrogen bond donors and acceptors, molecular weight, number of atoms, number of rotatable bonds and bioactivity scores (Kinase inhibitors, ion channel modulators, GPCR ligands, ion channel modulators, enzymes and nuclear receptors).
DPPH radical scavenging activity
The antioxidant activity (free radical scavenging activity) of the L-arginine derivatives on the stable radical DPPH (2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazy was determined according to the method described in literature [9,10]. The following concentrations of the derivatives were prepared in methanol. 500, 250, 125, 62.50, 31.25, 15.62, 7.8125, 3.91, 1.95 and 0.98 μg/ml. 2 ml of each concentration was mixed with 4ml of 50μM DPPH solution in methanol in triplicate. The mixture was vortexes for 10 seconds to homogenize the mixture and test tubes were incubated for 30 min at room temperature in the dark and the absorbance was measured at 515 nm using UV- V1S spectrophotometer (Shimadzu. 1620 Japan). Ascorbic acid was used as standard at the following concentrations 100, 50, 25, 12.5, 6.25, 3.125, 1.563, 0.7812, 0.391, and 0.195 M. Blank solutions were prepared by mixing 2ml of methanol with 4ml of 50 DPPH solution in methanol. The difference in absorbance between the test and the control (DPPH in methanol) was calculated and expressed as % scavenging of DPPH radical. The capacity of scavenge the DPPH radical was calculated by using the following equation:
% inhibition = 100 x (Abs control . - Abs .sample )/Abs control .
Where Abs control is the absorbance of DPPH solution and Abs controlsample is the absorbance of the sample after 30minutes
Results
Characterization of synthesized compounds
The structures of the synthesized compounds were established through FT1R, 1H-NMR and 13C NMR analysis.
Compound A1 -2-amino-5-{[(2,5-dioxo-2,5-dihydro-1H- pyrrol-1-yl)carbonoimidoyl]amino}pentanoic acid Yield- 86.65%, melting point- 110oC, Rf-0.65.
1R (KBr) cm-1: 3395-3239, 3330-2400, 2943.47,2359,2074, 1650, 1507.42,1364
1H NMR (400 MHz, Methanol-d4) 6.12 (s, 2H), 3.51 (d, J = 6.2 Hz, 1H), 3.08 (t, J = 6.9 Hz, 2H), 1.76 (ddp, J = 6.2Hz 2H), 1.61 (dd, J = 7.8, 16.1 Hz, 2H).
13C NMR (101 MHz), Methanol-d4) δ 173.21, 169.53, 157.20, 135.11, 53.93, 40.32, 27.77, 24.28. Compound A2- 2-amino-5-{[1,1-dioxido-3-oxo-1,2-benzothiazol-2(3H)-yl) carbonoimidoyl]amino}pentanoic acid. Yield- 60.91, melting point- 96oC, Rf-0.62.
1R (KBr) cm-1: 3340,3307.58, 1703.1,1065.27, 1654, 1215.
1H NMR (400 MHz, Methanol-d4) δ 7.87 - 7.75 (m, 4H), 7.80 - 7.66 (m, 2H), 3.36 (d, J = 8.6 Hz, 1H), 3.21 (s, 2H), 3.21 (d, J = 13.4 Hz, 2H), 1.81 - 1.67 (m, 2H), 1.71 - 1.62 (m, 2H).
13C NMR (101 MHz, Methanol-d4) δ 179.45, 170.94, 157.24, 144.06, 133.58, 132.40, 131.98, 123.02, 119.56, 55.24, 40.88, 31.27, 24.92.
CompoundA3-2-amino-5-{[(1,3-dioxo-1,3-dihydro-2H- isoindol-2-yl)carbonoimidoyl]amino}pentanoic acid
Yield-75.43%, melting Point- 88oC, Rf-0.72. FT1R (KBr) cm- 1 3402, 3482, 3189, 2958,1654,1540,1387,1282,1168,1084. 1H NMR (400 MHz, Methanol-d4) δ 8.07 (d, J = 3.5, 7.2 Hz, 2H,ArH), 7.92 - 7.71 (d, 2H, ArH), 3.64 (t, J = 6.1 Hz, 2H), 3.37 (s, 1H), 3.24 (td, J = 1.9, 7.0 Hz, 2H), 2.29- 1.92 (dtd, J = 3.8, 6.3, 9.3 Hz, 2H), 1.76-1.58 (ddt, J = 6.6, 9.7, 16.6 Hz, 2H). 13C NMR (101 MHz, Methanol-d4) δ 172.92, 171.79, 157.33, 134.26, 131.01, 130.44, 54.06,40.47,27.86,24.32(Tables 1-3)

a: Logarithm of partition Coefficient Between n-octanol and water (miLogPa); b: Topological Polar Surface area (TPSA); c: Molecular Weight (MW); d: Number of hydrogen bond acceptors (n-ON); e: Number of Hydrogen Bond Donors (n-OHNH); f: Number of Rotatable Bonds (n-rotb).


Discussion
Drug likeness determines if a particular molecule is similar to the known drug or not. It is a complex balance of different properties and structural features of a compound [11]. Lipinski's rule is mostly used to determine molecular properties that are vital for drug's pharmacokinetic behaviour According to Lipinski's rule of five, a compound is likely to be orally active if:
i. partition coefficient (log P) is less than 5.
ii. Hydrogen bond donor (OH and NH groups) is less tha 5.
iii. Hydrogen bond acceptor (N and O) is less than 10.
iv. Molecular weight is less 500 [12].
Partition coefficient (Log P) is a significant parameter used in drug design to determine molecular hydrophobicity or lipophilicity. Log P affects the absorption, bioavailability, drug- receptor interactions, metabolism and toxicity of molecules of a compound. Log P values of all the synthesized compounds (A1-A3) were found to be less than 5. This implies that these compounds will have good permeability across cell membrane. Molecular weight of all synthesized derivatives of L-arginine was found to be less than 500.
Topological polar surface area (TPSA) is closely linked to the hydrogen bonding potential of a molecule and is a very good predictor of drug transport properties like intestinal absorption and blood brain barrier penetration. TPSA of all the synthesized derivatives of L-arginine was found in the range of 138.28153.65 and is below the 160 limit. None of the synthesized compounds was found to be rigid as all of them had one or more than one rotatable bond. The synthesized compounds A1, A2 and A3 are flexible as they contain 7 rotatable bonds while L-arginine has 6.
The synthesized compounds had less than 10 hydrogen bond acceptors (O and N atoms) and the number of hydrogen bond donors (NH and OH) is less than 5. However L-arginine had 7 had hydrogen bond donors. It can be predicted that all the synthesized compounds are likely to be orally active as they did not violate any of Lipinski's rule of five.
As a general rule if the bioactivity score is large, the prospect that the investigated compound will be active is high. Therefore, a compound having bioactivity score more than 0.00 is most likely to have significant biological activities while values -0.50 to 0.00 are expected to be moderately active and if score is less than -0.50 it is presumed to be inactive. Bioactivity score for Enzyme inhibitor, GPCR ligand, ion channel modulator and protease inhibitor is found to be >0.00 for all tested compounds. This showed that the synthesized derivatives of L-arginine are biologically active molecules and will produce activity through various mechanisms.
The scavenging activity of the derivatives of L-arginine with compound codes A1, A2, A3 on DPPH are shown on Table 3. The result shows that the compounds had low DPPH antiradical activity with maximum 1C50 = 631.06μg/ml, 501.19μg/ml and 537.02μg/ml respectively as compared with that of ascorbic acid with 1C50 of 0.398μg/ml. This finding suggests that the guanidine group in L-arginine may be responsible for its antioxidant activity. Generally the presence of electron donor substituent such as alkyl group enhances the antioxidant property while electron withdrawing aryl group suppresses the DPPH scavenging ability.
Conclusion
The present study has established that the synthesized analogues of L-arginine showed low antioxidant activity compared to ascorbic acid based on 1C50 values however the analogues obeyed Lipinsky rule of five and showed good bioactivity scores.
References
- Singh S, Singh RP (2008) in methods of Assay of Antioxidants. Food Reviews International 24(4): 392-415.
- Freidovich 1 (1999) Fundamental aspects of reactive oxygen species, or whats the matter with oxygen? NY Acad Sci 893:13.?
- Yun-Zhong F, Sheng Y, GuoyaoWu (2002) Free radicals, antioxidants, and nutrition. Nutrition 18: 872-879.
- Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Review (4)8: 118-126.
- Morris SM (2006) Arginine: beyond protein. America Journal of Clinical Nutrition 83(2): 508S-512S.
- Wallner S, Hermetter A, Mayer B, Wascher TC (2001) The alpha-amino group of L-arginine mediates its antioxidant effect. European Journal of Clinical Investigation 31(2): 98-102.
- Adams MR, Phu CV, Stocker R, Celermajer DS (1999) Lack of antioxidant activity of the antiatherogenic compound L-arginine. Atherosclerosis 146(2): 329-335.
- Molinspiration Cheminformatics (2016).
- Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Food Science Technology 28: 25-30.
- Odumosu PO, Ojerinde SO, Egbuchiem M (2015) Polyphenolic contents of some instant tea brands and their anti-oxidant activities. Journal of Applied Pharmaceutical Science 5(09): 100-105.
- Khan SA, Kumar S, Maqsood A M (2013) Virtual Screening of Molecular Properties and Bioactivity Score of Boswellic Acid Derivatives in Search of Potent Anti-Inflammatory Lead Molecule. International Journal of Interdisciplinary and Multidisciplinary Studies 1(1): 8-12.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Advanced Drug Delivery Reviews 23(1-3): 3-25.






























